2030
Ketamine Induced Glutamate Alternations in Treatment-Resistant Depression. A 1H-MRS randomised placebo controlled study.1Neuroradiology, Karolinska University Hospital, Stockholm, Sweden, 2Clinical Neuroscience, MR Center, Karolinska Institutet, Stockholm, Sweden, 3Centre for Psychiatry Research, Karolinska Institutet & Stockholm Health Care Services, Region Stockholm, Stockholm, Sweden
Synopsis
Background: The glutamate N-methyl-D-aspartate receptor antagonist ketamine has a rapid antidepressant effect in major depressive disorder (MDD) patients. Though, the ketamine's possible mechanism of action has been debated the main hypothesis was focused around glutamate.
Methods: A proton short-echo MR Spectroscopy was utilised to examined three brain regions Anterior Cingulate Cortex, Hippocampus and Raphe nuclei before and 24-hours after treatment. 28 Selective Serotonin Reuptake Inhibitor resistant MDD patients were randomized to double blind monotherapy with 0.5 mg/kg ketamine or placebo infusion.
Results: glutamate correlates with depression symptoms severity in ACC and HC in opposing ways in ketamine group.
Introduction
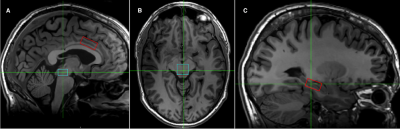
Administration of ketamine at a sub-anesthetic dose has brought about a paradigm shift in the treatment of major depressive disorder (MDD). The therapeutic effects within hours rather than weeks and with long duration of effect has been observed(1). While a mechanism of action of ketamine in MDD treatment has still not been determined the glutamate hypothesis has dominated in the field(2). The ketamine seems has two competing effects that are well separated in time. Firstly, ketamine induces the dissociative antagonistic effects via glutamate NMDA receptors. This implies that the synaptic extracellular glutamate would increase – an effect which may be short lived. Thereafter, the rapid growth of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors was suggested as a result of remodeling of the post-synaptic cleft(2). An excess of glutamate could utilize the availability of new AMPA receptors that may result in the decrease of synaptic glutamate concentration. The aim of the study was to explore the glutamate and the other metabolite concentrations changes after 24 hrs of one subanestetic dose of ketamine or placebo in Selective Serotonin Reuptake Inhibitor resistant (SSRI) MDD patients. The study was focused on the three brain regions (Fig. 1): the dorso-medial anterior cingulate (dACC), the hippocampus (HC) and the dorsal raphe nucleus (dRN).Methods
The MR scanning was performed on a 3 Tesla scanner (MR750, GeneralElectric) using 8-channels head receiver array (InVivo). MRS-PRESS localization with TR/TE/TE1= 2000/30/12 ms was used with total NEX 128 (dACC), 224 (HC&dRN). Voxel B0 field inhomogeneity adjustment was performed by using the primary gradient coils. The high-order shims did not add any improvements for small voxels. To enhance repeatability hence, precision of repeated measures the following were undertaken: (i) the voxels were positioned using defined anatomical landmarks found on 3D T1w image, (ii) the voxel positions were refined before every MRS scan by newly acquired three-plane localiser images (iii) between two visits for the same patient the transmitter gain was controlled and if differs was set manually.MRS data quantification was performed by using LCModel-v.6.3-1K(3). Data was pre-processed in Matlab, FID-A toolbox(4). The pre-processing includes: snr-weighted signal coil combining, frequency and phase correction for every MRS trace and corrupted trace-removal based on the tCr peak fidelity filters. The basis set for 20 metabolites was simulated using the actual parameters used in pulse sequence. Data was quantified wrt. total creatine. Concentrations are presented in mM assuming average total creatine concentration 7 mM.
Patients were randomized to study treatment with racemic ketamine or saline (2:1). Active treatment was 0.5 mg/kg racemic ketamine diluted in 100 ml isotonic NaCl solution, given as an intravenous infusion during 40 minutes. Placebo treatment was isotonic NaCl solution. The severity of depression symptoms was rated with Montgomery–Åsberg Depression Rating Scale (MADRS) and MADRS-short was also used. Response to treatment was quantified as a reduction of more than 50% of the MADRS score from the baseline.
Experiment time-line:
day0(MRS,MADRS) >>24-72h>> Treatment(MADRS) >>24h>> day1(MRS,MADRS)
Study Participants
The study was approved by the Regional Ethical Review Board. The inclusion criteria were: ongoing major depressive episode (MADRS≥20), treated for at least four weeks with SSRI without treatment response. The exclusion criteria were: medication for the ongoing depressive episode, bipolar disorder, psychosis, other comorbid disorder as primary diagnoses. Patients in ketamine/placebo group were 19/9. Mean duration of current depressive episode was 48 months. The mean age, gender, BMI were group-matched.Results
The visual quality check of all quantified spectra led to exclusion of one subject from further analysis. The QA metrics were very similar between two visits. The statistical Kruskal-Wallis non-parametrical testing between day0 vs. day1 revealed none significant differences.Results for baseline (day0)
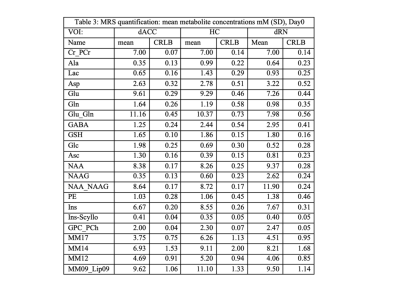
The metabolite concentrations are shown in Table 1 for all MDD patients. A statistical linear regression with “MADRS_short” as a predictor revealed:
ACC: GABA and glutathione but not Glu has shown negative correlation (GABA: p<0.032, r.sq=0.18, GSH: p=0.0337, r.sq.=0.17). Inositol has increased (p=0.035, r.sq=0.16), NAAG had increasing trend (p=0.08 r.sq.=0128).
HC: none significant correlation.
dRN: inositol (p=0.0126, r.sq.=0.24) and aspartate (p=0.05, r.sq.=0.148) were decreased significantly.
Differential response to ketamine & placebo treatment
There were 8 responders, 6 delayed responders and 5 non-responders (26%) in ketamine treatment group (N=19), 24h. post-infusion. Two placebo patients were convinced they were taken ketamine. The individual metabolic trends for day0-day1 were randomly distributed between positive, negative and no-change (Fig.2). The group analysis of mean (ANOVA) or ranks (non-parametric tests) did not provide any conclusive results. There only MADRS scores that differ significantly between treatment groups Pla:24(±3); Ket:16.6(±1.6) p<0.00001. Therefore, statistical linear regression was performed on relative differences (%Met.Concentration=day1-day0/day1) vs. MADRS-short_%change for Placebo and Ketamine.
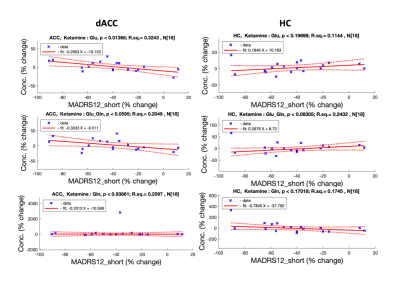
dACC ketamine_group has shown significant glutamate increase with depression symptoms improvement (Glu-ketamine: p=0.0136, r.sq=0.32, Glu-placebo: p=0.99, r.sq.=0.0). see Fig 2.
HC: glutamate decrease with improving depression symptoms was observed in the ketamine group (Glu-ketamine: p=0.019, r.sq.=0.418, Glu-placebo: p=0.1, r.sq.=0.32). see Fig 2.
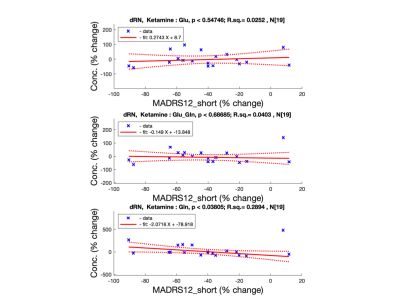
dRN: only glutamine has positive correlation with patient improvement for ketamine treatment group (Ketamine: p=0.038, r.sq.=0.289; Placebo: p=0.78, r.sq.=0.016) see Fig 3.
Discussion & Conclusions
The ketamine induced Glu-increase in ACC and Glu-decrease in HC, both correlated with depression symptoms may suggest a highly competitive and time-shifted balance between AMPA and NMDA receptors in these brain regions.Acknowledgements
This study was supported by Region Stockholm (ALF project 20170192 and higher clinical research appointment).
The staff at Hjärnstimuleringsenheten, Norra Stockholms Psykiatri, Stockholm, is acknowledged for randomization, dosing and screening of patients.
The staff at Karolinska MR Centrum is acknowledged for hosting the project including MR data collection, data management and patient's clinical data evaluation.
References
1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000): Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354.2.
2.Mathew SJ, Zarate, CA (Eds.) (2016): Ketamine for Treatment-Resistant Depression. Cham: Springer International Publishing.
3.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9. doi: 10.1002/mrm.1910300604.
4.Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID‐A)—An open source, MATLAB‐based toolkit. Magn Reson Med. 2017 Jan 1;77(1):23-33.
Figures