2022
T1 Weighted Postmortem MR Imaging of the Cerebellum at 3T: Preliminary Results between Feasibility and Desire1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, Faculty of Medicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurological Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland, 3Dept. of Radiology, Division of Radiological Physics, University Hospital Basel, Basel, Switzerland, 4Department of Cognitive Neurology, MR-Research in Neurology and Psychiatry, University Medical Center Göttingen, Göttingen, Germany, 5Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany, 6Translational Neuroradiology Section, Division of Neuroimmunology and Neurovirology, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
Synopsis
MRI of the fixed human brain is highly interesting, since it basically allows very long scan times for unprecedented MRI resolutions on clinical scanners. Recent work utilized T2* weighted RF spoiled gradient echo sequences to achieve isotropic resolutions up to 160-microns at 3T. This work establishes a T1 weighted MR imaging protocol based on RF spoiled gradient echo sequences that currently enables isotropic resolutions of 240-microns at 3T and depicts the complex fine structure of the fixed cerebellum very well.
Introduction
MR imaging of the ex vivo healthy and diseased human brain offers the great potential for a deeper understanding of neuro morphology and pathology 1-5. As shown in recent studies, T2* weighted RF spoiled gradient echo (FLASH) sequences with very low receiver bandwidth offer the potential of performing ultra-high resolution imaging (URI) of the entire human brain at 3T and 7T field strength with a quality not achievable under in vivo conditions 4,5.The purpose of this work was to develop a T1 weighted (T1w) URI-FLASH approach for a clinical 3T MR system that is especially dedicated to the cerebellum. Considering the fine details of the cerebellum and the application of a clinical 3T MR system with standard hardware only, this desire may almost seem “outrageous”. Nevertheless, we can show that impressive images can be acquired within the time of approximately 28h, which corresponds to a full day and a long night of continuous measurement.
Methods
Brain preparation and Experimental setupThe brain of a patient with secondary progressive MS was fixed in 4% formalin approx. 24h after death. For MRI acquisition, the brain was positioned in a dome-shaped container as depicted in Refs. 6-8 and immersed in Fomblin, a fluorinated oil. Air bubbles were aspirated through the spout of the container through a vacuum pump. All acquisitions were performed with a 3T whole-body MR system using the standard 20-channel phased-array head coil.
Acquisition
In a pre-experiment, a fast T1 mapping approach 9 was used to determine the approximate range of T1 in the present brain (cerebellum) to tune the excitation flip angle.
For the T1w URI-FLASH approach, we followed the rationale by Weigel et al. 5 and harnessed an in-house developed RF spoiled gradient echo sequence that circumvents typical restrictions like maximal 3D matrix size and, thus, can use the full vendor’s available memory and on-the-fly reconstruction capability.
Two different base protocols were set up, focusing on the cerebellum: (1) Isotropic resolution (270μm)3, matrix 512x512x512, 17% phase oversampling, 25% slice oversampling, transverse slices with readout in A-P direction, TR/TE=18.0ms/8.2ms, bandwidth=110Hz/Px, TAbase=01:55:12h, 2 repetitions for averaging; (2) isotropic resolution (240μm)3, matrix 496x546x512, 40% phase oversampling, 25% slice oversampling, transverse slices with readout in A-P direction, TR/TE=19.0ms/8.4ms, bandwidth=110Hz/Px, TAbase=02:35:02h, 11 repetitions for averaging. The excitation flip angle of 77° was chosen far beyond the expected Ernst angle 10 to introduce a strong T1 weighting.
For image reconstruction, solely the standard MR system reconstruction was used: no kind of filtering, no interpolation and absolutely no image registration concepts.
Results
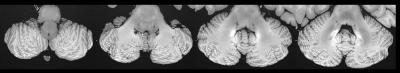
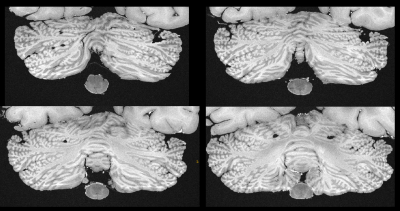
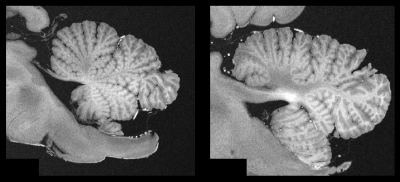
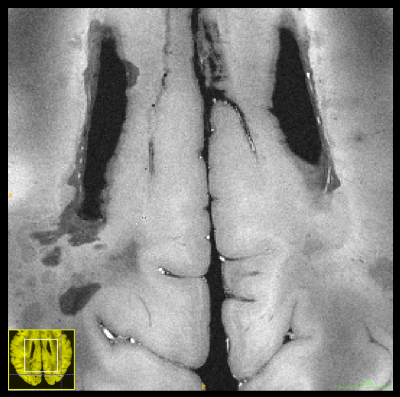
Figure 1 contrasts acquisitions with the two base protocols of 270µm and 240µm isotropic resolution, including a comparison of single acquisitions with averaged, repeated measurements. Despite the long total acquisition time (28h), images are artifact-free and do support the nominal resolution as specified. Figure 2 presents a further selection of transverse slices that depict the complex fine structure of the cerebellum. Figure 3 illustrates the benefit of coronal reformations. Two zoomed images in a sagittal reformation demonstrate the cerebellum in a nice overview (Figure 4). However, they are also meant to demonstrate the current limitation of the approach with 11 averages performed.Additionally, Figure 5 displays an example for a transverse slice around the ventricles, also displaying some lesions.
Discussion and Conclusion
Recent FLASH based postmortem URI approaches produced valuable and fascinating images of the human brain, which were T2* weighted 4,5. Our URI-FLASH protocol provides a strong T1 weighting and leads to a precise delineation of fine structure in the cerebellum.The published T2* weighted URI FLASH approaches make use of bandwidths as lows as 50Hz/Px 4,5, which leads to a significant SNR enhancement and, thus, increase in acquisition efficiency. The present T1w URI-FLASH approach can only use this methodology to a limited extent, because it avoids using low echo times that are needed for a predominant T1 contrast. Thus, the used TE=8.4ms can be considered as a trade-off between SNR enhancement based on a low bandwidth and the resulting T1 contrast.
For the future, a further increase in spatial resolution is planned. Based on the investment of an entire weekend for acquisition, an increase to an isotropic resolution of ca. 210µm can be reasonably estimated, while at least maintaining the present SNR. This further potential increase of ca. 30µm in isotropic spatial resolution may seem like a small benefit, however, as Weigel et al. 5 already showed, cerebellar imaging should benefit from it. Moreover, it should be considered that, generally, increases in MRI spatial resolution necessitate notable SNR reserves.
To conclude, T1w URI-FLASH offers ultra-high resolution MR imaging of the fixed cerebellum with a strong well-delineated T1 contrast, and only necessitates a standard 3T clinical MRI system without the need for any specialized hardware.
Acknowledgements
This work was funded by the Swiss National Fund PP00P3_176984 and supported by the German Ministry of Education (BMBF; KKNMS German competence network for multiple sclerosis). Govind Nair is supported by the Intramural Research Program at the NINDS.References
1. Pfefferbaum A, Sullivan EV, Adalsteinsson E, et al. Postmortem MR imaging of formalin-fixed human brain. Neuroimage 2004;21(4):1585-95.
2. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 2011;57(1):167-181.
3. Tendler BC, Hanayik T, Ansorge O, et al. The Digital Brain Bank, an open access platform for post-mortem datasets. bioRxiv preprint doi: https://doi.org/10.1101/2021.06.21.449154
4. Edlow B, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data 2019 Oct 30;6(1):244.
5. Weigel M, Dechent P, Galbusera R, et al. Imaging multiple sclerosis pathology at 160μm isotropic resolution by human whole-brain ex vivo magnetic resonance imaging at 3 T. Sci Rep 2021 Jul 29;11(1):15491. doi: 10.1038/s41598-021-94891-1.
6. Luciano NJ, Sati P, Nair G, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp 2016 Dec 6;(118).
7. Absinta M, Nair G, Filippi M, et al. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J Neuropathol Exp Neurol. 2014;73(8):780-8.
8. Griffin AD, Turtzo C, Parikh G, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142(11):3550-3564.
9. Wang X, Roeloffs V, Merboldt KD, et al. Single-shot Multi-slice T1 Mapping at High Spatial Resolution – Inversion-Recovery FLASH with Radial Undersampling and Iterative Reconstruction. The Open Medical Imaging Journal 9(1):1-8
10. Ernst RR. Application of Fourier transform spectroscopy to magnetic resonance. Review of Scientific Instruments 1966;37: 93.
Figures
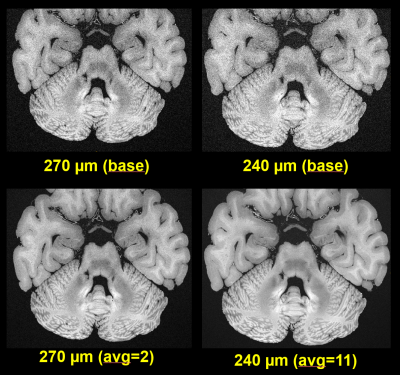
The measurements display the original FOV. Resolution and number of averages as noted.