2021
Investigating Early Brain Development and Executive Function in Young Children1Waisman Center, University of Wisconsin-Madison, Madison, WI, United States, 2Neuroscience Training Program, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Medical Physics, University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, United States, 4Department of Psychiatry, University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, United States, 5Department of Pediatrics, University of Wisconsin-Madison School of Medicine and Public Health, Madison, WI, United States
Synopsis
The emergence of executive function (EF) in children is an important developmental process that impacts later cognitive and behavioral outcomes; however, much remains unknown about the neural processes underlying the development of EF. In this study, we quantified volumetric measures from structural MRI to investigate associations between EF and brain structure in children aged 3 to 10 years old. Results indicated that there were correlations between EF and structures in subcortical brain regions and regions of the frontal and parietal lobes.
Introduction
Executive Function (EFs) are a set of higher-order processes involved in the conscious control of thoughts and actions, particularly goal-directed behaviors1. In preschool-aged children (ages 3 to 6 years), EF presents as a unitary construct2,3. Beyond preschool, there is evidence that EF processes such as working memory and response inhibition become more differentiated as development progresses into late childhood and adolescence3,4. This implies that drastic structural changes in the brain occur during early childhood as EF develops and diversifies. The frontal lobes, prefrontal cortex, subcortical structures, and parietal cortex are known to be involved in EF processes5,6. We combined data from the NIH Toolbox-Cognition Battery (NIHTB-CB)7 and structural magnetic resonance imaging (MRI) and evaluated correlations between regional brain measurements and performance on three NIHTB-CB tasks to determine the regions of the brain that are key to the development of EF in young children8. We hypothesized that higher brain structural measurements would correlate with better performance on the NIHTB-CB measures of EFs.Methods
Children who enrolled in the study and completed both the MRI scan and NIHTB-CB sessions (n=26, 15 males) were between 3 and 10 years of age (average age = 6.7 years, 3.5 years of school completed). The mothers in the study were highly educated, having completed between 20 and 24 years of education. Exclusionary criteria included, for the child, a history of any brain injuries or illnesses, psychiatric diagnoses, major birth defects and malformations, cardiac disease, cancer and any contraindications to MRI, and for the mother, a history of bipolar, schizophrenia or PTSD diagnoses, brain injuries or illnesses, cancer and major injuries, illnesses or infections during pregnancy. A General Electric (GE) MR750 3 Tesla and Nova 32-channel head coil were utilized for MRI acquisition. An MPnRAGE T1-weighted image was acquired for each participant9 and automatically segmented using the FreeSurfer Image Analysis suite version 7.1.110,11. Measures of brain volume, cortical thickness, and surface area from regions in the frontal lobes, parietal lobes, and subcortical structures were used in subsequent analyses. Three tests from the NIHTB-CB formed our cognition battery: The Flanker Inhibitory Control and Attention Test, Dimensional Card Sort Test, and List Sorting Working Memory Test. A composite EF score was computed for each individual by averaging their uncorrected standard scores on the NIHTB-CB tasks. We used RStudio to examine associations between EF score and structural measurements for our regions of interest. We corrected for age and sex when examining surface area and cortical thickness, while corrections for age, sex, and estimated total intracranial volume were used for cortical and subcortical volumes.Results
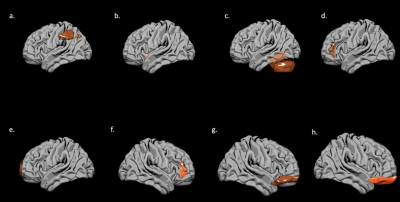
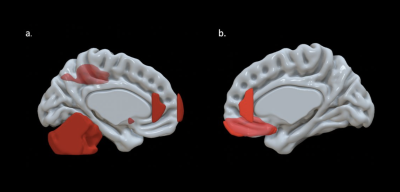
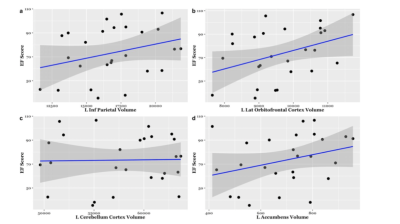
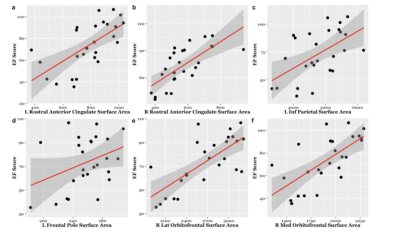
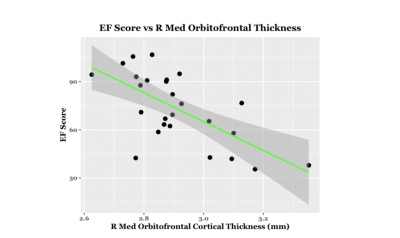
EF scores correlated with neuroimaging measures across the brain (Fig. 1, Fig. 2). Specifically, EF scores correlated with the following: The cortical volumes of the left inferior parietal (LIPC) and right lateral orbitofrontal cortexes (RLOFC) (Fig. 3), the subcortical volumes of the left accumbens and left cerebellum cortex (Fig. 3), the surface areas of the left rostral anterior cingulate cortex (LRACC), left inferior parietal cortex (LIPC), left frontal pole (LFP), right rostral anterior cingulate cortex (RRACC), right lateral orbitofrontal cortex (RLOFC), and right medial orbitofrontal cortex (RMOFC) (Fig. 4), and the cortical thickness of the right medial orbitofrontal cortex (RMOFC) (Fig. 5). The significant association between the surface area of the RLOFC and EF score survived multiple comparisons corrections (p = .048).Discussion
There were multiple significant relations between brain measurements and the composite EF score. All volumetric and surface area measurements were positively correlated with the composite EF score, suggesting increases in brain volume and surface area are related to increasing EF ability. On the other hand, measures of thickness were negatively correlated with EF, indicating improved EF with decreasing cortical thickness. Interestingly, we observed associations in the right hemisphere, which could suggest hemispheric specialization. The RLOFC helps integrate sensory information, coordinate response inhibition, decision-making, and communication with the limbic system, all of which are integral to EF12. Results showed significant trends in multiple other regions of interest involved in EF processes, including brain regions dedicated to the integration of sensory information (LIPC)13; communication between the frontal lobe and subcortical structures (LRACC, RRACC)12; response inhibition and memory updating (LRACC, RRACC)13; integration of EF with emotional processes (LFP)14; motivation and inter-region communication (left accumbens)5; and regulation of motor tasks (left cerebellum)5. Although many of these associations did not survive multiple comparisons correction, they show promise for future exploration. The main limitation of this study is the small sample size. Due to the restrictions caused by the COVID-19 pandemic, recruitment for in-person study visits was difficult. These limitations made us unable to look at EF related to both age and brain measurements. Further investigation will involve expanding the overall sample and investigating age-related associations between EF and brain measurements.Conclusion
Neural architecture is implicated in variability in children’s EF. Identifying brain regions associated with the emergence and development of EF during early childhood is important for understanding the neurodevelopmental processes related EF development. Clarifying the neural mechanisms underpinning the development of EF in typically-developing children could advance our understanding of the sensitive period for EF development, and thus provide a framework for studying EF in atypically-developing populations.Acknowledgements
We would like to thank our research participants and their families who participated in this research as well as the dedicated research staff who made this work possible. We acknowledge GE Healthcare, which provides research support to the University of Wisconsin. This work was supported by grants R34 DA050258 (Dr. Alexander) from the National Institute of Drug Abuse; R00 MH11056 (Dr. Dean) from the National Institute of Mental Health, National Institutes of Health. Infrastructure support was also provided, in part, by grant U54 HD090256 from the Eunice Kennedy Shriver NICHD, National Institutes of Health (Waisman Center).References
1. Miyake A, Friedman N, Emerson M, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cog Psychol. 2000;41: 49-100.
2. Weibe S, Sheffield T, Mize Nelson J, et al. The structure of executive function in 3-year-old children. J Exp Child Psychol. 2011;108(3): 436-452.
3. Akshoomoff N, Brown T, Bakeman R, et al. Developmental differentiation of executive functions on the NIH Toolbox Cognition Battery. Neuropsychology. 2018;32(7): 777-783.
4. McAuley T, White D. A latent variables examination of processing speed, response inhibition and working memory during typical development. J Exp Child Psychol. 2011;108(3): 453-468.
5. Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychologica. 2004;115: 271-289.
6. Zelazo P, Anderson J, Richler J, et al. NIH Toolbox Cognition Battery (CB): Measuring executive function and attention. Monographs of the Society for Research in Child Development. 2013;78(4): 16-33.
7. Hodes RJ, Insel TR, Landis SC; NIH Blueprint for Neuroscience Research. The NIH toolbox: setting a standard for biomedical research. Neurology. 2013;80(11 Suppl 3): S1.
8. Gershon R, Wagster M, Hendrie H, et al. NIH Toolbox for assessment of neurological and behavioral function. American Academy of Neurology. 2013;80(3): S2-S6.
9. Kecskemeti S, Samsonov A, Hurley S, et al. MPnRAGE: A technique to simultaneously acquire hundreds of differently contrasted MPRAGE images with applications to quantitative T1 mapping. Magn Reson Med. 2016;75(3): 1040-1053.
10. Dale, A.M., Fischl, B., Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9: 179-194.
11. Fischl, B., Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97: 11050-11055.
12. Elliot R, Dolan R, Frith C. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3): 308-317.
13. Salmon E, Van der Linden M, Collette F, et al. Regional brain activity during working memory tasks. Brain. 1996;119: 1617-1625.
14. Stuss D. Functions of the Frontal Lobes: Relation to Executive Functions. J Int Neuropsych Soc. 2011;17: 759-765.
15. "NITRC: Surf Ice: Tool/Resource Info." NITRC, https://www.nitrc.org/projects/surfice/.Figures