2020
A Neurochemical Atlas of the Cingulate Cortex in Relation to Pain.1Experimental Medicine, University of British Columbia, Vancouver, BC, Canada, 2Radiology, University of British Comulbia, Vancouver, BC, Canada, 3Philips, Vancouver, BC, Canada, 4Wissenschaftlicher Mitarbeiter Forensische Medizin und Bildgebung, Universität Zürich, Zürich, Switzerland, 5Anesthesiology, Pharmacology and Therapeutics, University of British Columbia, Vancouver, BC, Canada
Synopsis
1H-MRS provides a window into brain chemistry without the need to directly sample tissue. The growing prevalence of neurological conditions highlight the need for evidence based treatments. Specifically, preclinical and clinical evidence suggest a link between glutamate and pain. This study shows spatial neurochemical differences from different voxels across the cingulate cortex using sLASER at 3 T. A significant correlation between pain ratings and the anterior cingulate cortex is seen. As MRS moves towards becoming a standardized clinical technique, understanding the spatial distributions and variability will provide an important basis for the understanding of the healthy human brain’s biochemical environment.
Introduction
Neurological conditions are a growing global concern and evidence-based treatments for these represent a priority area to strengthen the healthcare system. Central to evidence-based treatments are advanced neurophysiological techniques. These are used to examine neurological conditions including neurodegenerative (e.g., Alzheimer's) and psychiatric conditions (e.g., schizophrenia), and clinical pain (e.g., Fibromyalgia). Measurement of neurochemicals via proton-magnetic resonance spectroscopy (1H-MRS) provides a non-invasive, unique opportunity to examine the brain's biochemical environment. Emerging from animal and human studies there is evidence to suggest a link between glutamate and pain1–4. The cingulate cortex, consisting of the anterior, medial, and posterior divisions, is a part of the limbic system that, is consistently activated during pain, but the functional specificity of cingulate divisions has not been considered in human pain experiments 5. The distinct cingulate subdivisions can be delineated based on neurochemistry to gain insight into biochemical processes in health and disease. We have performed a metabolic map of the cingulate cortex using MRS localized with sLASER at 3T and examined the relationships to measures of pain sensitivity.Methods
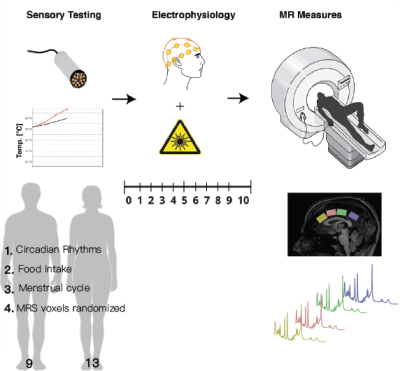
ParticipantsTwenty-two healthy participants were recruited for this experiment (9M/13F, mean age= 26.1 SD= 4.8 range=19-35). Participants were asked to come to the laboratory in a fasting state. The timing of the scan was kept consistent (11:30 am-12:30 pm) across participants and women were tested approximately during the follicular phase of their cycle Figure 1.
Pain Measurements
Quantitative sensory testing involved collecting participants' pain thresholds (method of limits, 32°C, ramp rate 1 °C/s, 30s ISI). Four trials were performed, with the final threshold determined as an average of the final three6. Pain sensitivity was measured with laser evoked potentials (LEPs), the participants were then fitted with an EEG cap and LEPs were performed (total 80 stimuli); the participants rated each stimulus using the 0-10 numeric pain rating scale. Participants were then given a muffin and escorted to the MRI center Figure 1.
MR Procedures
3T data were collected on a 3T Philips Ingenia Elition X, with a 32 channel SENSE head coil, and the sequences included:1) MPRAGE 3D T1 (TE/TR/TI=4.3/9.3/950ms, shot interval=2400ms, +0.8mm³ isotropic resolution, FOV (ap/rl/fh) =256/256/180mm³, scan time=5:49).2) 1H-MRS (sLASER, TE/TR=32/5000ms, NSA=64, voxel size=30/25/15mm3 =11.2mL, automated 2nd order shimming, 32-step phase cycle with water suppression using the frequency selective Excitation option. Four voxels were prescribed along the AP direction in the cingulate cortex (Figure 1), and the order of MRS acquisition in the voxels was randomized for each participant.
Data Analysis
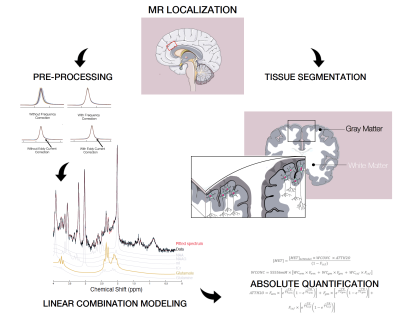
Figure 2 shows the MRS analysis steps which included, pre-processing and absolute quantification performed using in-house MATLAB scripts. Spectral fitting was performed with LCModel version 6.37. A liner-mixed model and pairwise comparisons for each location were performed in R Studio (V1.1.442- lme4 Bates Maechler & Bolker, 2012). P-values were adjusted for comparing four estimates.
Results
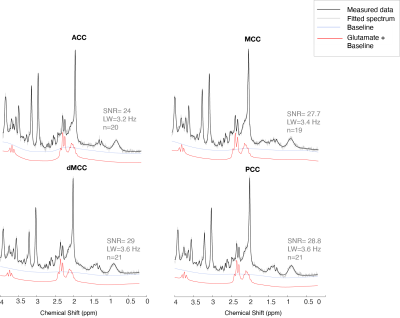
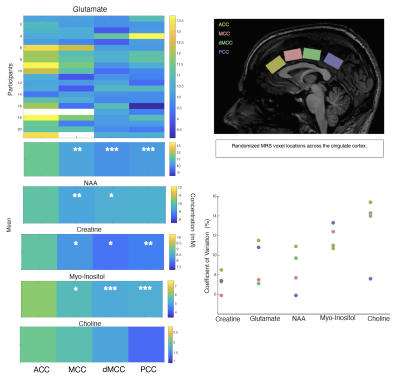
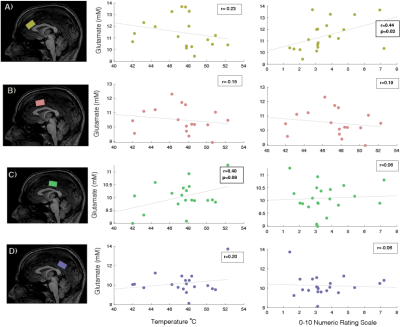
One participant’s data had to be rejected, and for three other participants, we excluded metabolites from one location, due to data quality. The data quality form included participants can be seen in Figure 3 and the SNR and LW were similar across locations. The missing values can be seen in white in Figure 4. No sex differences were seen. Significant differences in metabolite concentrations were seen for glutamate (ACC - MCC β=0.97±0.28, p=0.005; ACC –dMCC β=1.34±0.27, p= <.0001; ACC-PCC β= 1.17±0.27, p=0.0004), NAA (ACC - MCC β=0.69±0.19, p=0.003; ACC–dMCC β=0.521±0.18, p=0.03), creatine (ACC-MCC β=0.67±0.23, p=0.02; ACC-dMCC β=0.74±0.22, p=0.01; ACC – PCC β=0.82±0.23, p=0.004) and myo-inositol (ACC - MCC β=0.67±0.21, p=0.01; ACC – dMCC β=1.02±0.20, p=<.0001; ACC – PCC β=0.97±0.20, p=0.0001). While choline remained constant across locations (Figure 4). A Pearson correlation revealed a significant relationship between glutamate levels and pain ratings in the ACC and a trend for pain thresholds and glutamate in the dMCC (Figure 5). The coefficient of variation of the neurochemicals per location can be seen in Figure 5.Discussion & Conclusion
The relationship between glutamate concentrations and pain outcomes in the cingulate subdivisions reveal intriguing results fitting into an existing framework set out by Rohini Kuner’s group whereby a pathway from the MCC to the posterior insula has been demonstrated to gate adaptive sensory responses without influencing pain affect5. Whereas, the ACC has been recognized for its relationship with cognitive appraisal or pain and fear. These results suggest there may be functional specificity of MRS-visible neurochemicals in the subdivisions of the cingulate cortex. As MRS moves towards becoming a standardized clinical technique, understanding the spatial distributions and variability across regions and metabolites will provide an important basis for the understanding of the healthy human brain’s biochemical environment. These findings will be used as a basis for a planned clinical pain (i.e., Fibromyalgia) cohort where the same methodology will be applied. Practically, these results can inform other studies in decision-making regarding voxel placement depending on the respective research question.Acknowledgements
We would like to thank the University of British Columbia MRI Research Centre, the International Collaboration for Repair Discoveries (ICORD), and volunteers who participated in the study.
J. Archibald is supported by a research scholarship of the National Council of Science and Technology (CONACYT), The Natural Sciences and Engineering Research Council of Canada (NSERC) Top Doctoral Scholarship, and the Friedman for Health Scholars foreign study award.
E.L MacMillan receives salary support from Philips Canada.
This work is supported by NSERC Program Discovery grant held by JLK Kramer.
References
1. Malmberg, A. B. & Yaksh, T. L. The effect of morphine on formalin‐evoked behaviour and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br. J. Pharmacol. (1995) doi:10.1111/j.1476-5381.1995.tb13315.x.2. Sluka, K. A. & Willis, W. D. Increased spinal release of excitatory amino acids following intradermal injection of capsaicin is reduced by a protein kinase G inhibitor. Brain Res. 798, 281–286 (1998).3. Archibald, J. et al. Excitatory and inhibitory responses in the brain to experimental pain : A systematic review of MR spectroscopy studies. Neuroimage 215, 116794 (2020).4. Archibald, J. et al. Metabolite activity in the anterior cingulate cortex during a painful stimulus using functional MRS. Sci. Rep. 10, 1–12 (2020).5. Tan, L. L. et al. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat. Neurosci. 20, 1591–1601 (2017).6. Engen, T. Psychophysics I: Discrimination and detection. Woodworth Schlosberg’s Exp. Psychol. 11–46 (1971).7. Provencher, S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30, (1993).Figures