2018
Assessment of subtle BBB disruption using Focused Ultrasound: comparison between the contrast agent exchange rate and the water exchange rate1Vilcek Institute of Biomedical Science, New York University School of Medicine, New York, NY, United States, 2Radiology, Center for Advanced Imaging Innovation and Research, New York, NY, United States, 3Radiology, Weill Cornell Medical College, New York, NY, United States
Synopsis
This study explores two different approachs of measuring subtle BBB disruption. To induce different levels of BBB disruption, we used a focused ultrasound (FUS) sonication with intravenously injected microbubbles with an animal model. Each animal underwent FUS procedure with different acoustic power levels. We compared the changes measured using DCE-MRI with Gadolinium-based contrast agent for volume transfer rate constant and Ferumoxytol-based ACE-MRI to measure transendotheliel water exchange rate. Our results suggest that both the water exchange rate and the contrast exchange rate show sensitive detection of subtle BBB disruption, which could shed light on understanding different permeability changes in BBB.
Purpose
Dynamic contrast enhanced (DCE)-MRI has been widely used as a quantitative tool for assessing the subtle blood-brain barrier (BBB) disruption, suggested as a potential biomarker for early Alzheimer’s disease [1]. However, due to this subtle disruption, the extravasation of Gadolinium contrast agent is expected to be also small, which in turn requires a relatively long scan time to observe [2]. Ferumoxytol has recently gained attention as an alternative contrast agent for MR imaging [3, 4]. Due to its large molecular size, Ferumoxytol molecules remain in blood vessels with subtle BBB disruption. Utilizing its paramagnetic characteristics of T1-shortening effect on nearby water, we aim to measure the water exchange rate between the intravascular and extravascular space. We compare the contrast agent exchange rate and the water exchange rate for the assessment of different levels of BBB disruption induced by Focused Ultrasound (FUS).Method
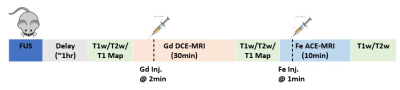
Focused Ultrasound: The Sprague Dawley Rats (Female, n=5, 6-8 weeks old, body weight= 250-350g) underwent Stereotactic Focused Ultrasound procedures with different acoustic power. The animals were injected with Optison microbubbles via a tail vein catheter while transmitting the ultrasound wave. This procedure is known to temporarily induce BBB disruption by sonicating the microbubbles in the vessels [5]. Each rat received different acoustic power levels ranging from 0.2 to 1.0MPa with an increment of 0.2MPa, prior to MRI scan as shown in Figure 1.DCE-MRI with Gadolinium-based contrast agent: A 3D golden-angle radial sampling sequence with ultrashort echo time was employed to acquire dynamic scans. The Gadolinium-based contrast agent (gadobutrol, 0.1mMol/kg) was injected at 2min into the scan via tail-vein catheter. The total scan time for DCE-MRI was 28min. The imaging parameters are TE/TR = 0.028 / 10ms, flip-angle = 10°, spatial resolution = 0.203x0.203x0.203mm3, the image matrix = 128x128x128. The acquired image was reconstructed with iterative GRASP reconstruction with the temporal resolution of 5s [6]. Prior to DCE-MRI scan, similar 3D radial sequence was employed to acquire pre-contrast T1 maps using 3 different flip-angles.
ACE-MRI with Ferumoxytol-based contrast agent: The DCE-MRI scan with Ferumoxytol injection was conducted using the Active Contrast Encoding MRI (ACE-MRI) sequence that uses multiple flip angles to encode the effect of water exchange effect on the dynamic signal intensity time curve. ACE-MRI was conducted with the 3D radial sequence with ultrashort echo time (TE = 0.028 ms) with a series of 14 segments with different flip angles in the order of [10°-10°-10°-10°-2°-10°-20°-10°-5°-10°-15°-10°-25°-10°]. The diluted Ferumoxytol (0.4 – 1.6mg Fe / kg) was injected at 1min into the scan and the scan continued for another 9min. The images were reconstructed with 2.5s temporal resolution.
Data Analysis: For DCE-MRI data with gadobutrol, the pharmacokinetic model analysis was performed using the graphical Patlak model [7]. The arterial input-function (AIF) was sampled from the image by selecting the top 0.5% enhancing voxels. The permeability-surface area product (PS) was obtained to assess the exchange rate for Gadolinium contrast agent. For ACE-MRI data with ferumoxytol, the extended Tofts model [8] was used for modeling the vascular and extravascular compartment. Since ferumoxytol molecules are expected to remain in the vascular compartment, the contrast exchange rate (PS) was assumed to be negligible (i.e., PS=0). Then the signal rising from the water exchange between the intravascular and extravascular compartment was modelled as similar to the previous study [6], with the absence of transcytolemmal water exchange. The sonicated region was identified using the PS map from Gd and selected as ROI for fitting the model. The mean residence time of water denoted as τb in the ROI was estimated for assessing the water exchange rate at the sonicated region.
Results
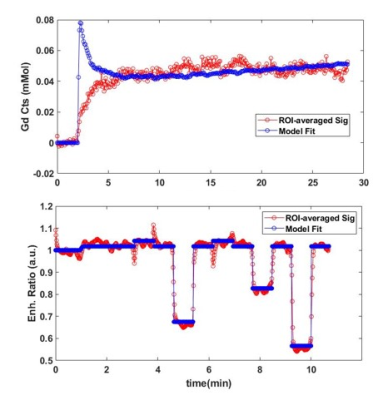
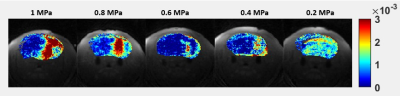
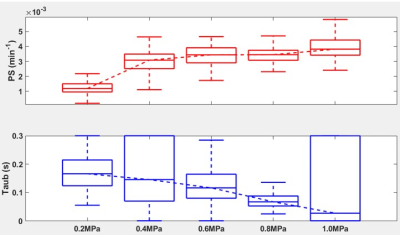
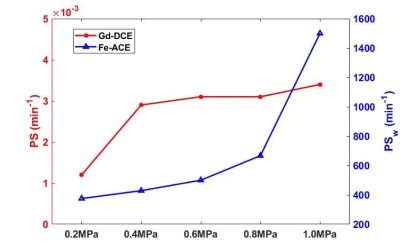
Figure 2 shows an example fit of each model for DCE and ACE-MRI in the ROI-averaged signal. As shown, each model was able to describe the dynamics of both contrast agents. In Figure 3, the PS map obtained from the DCE-MRI data are displayed for different acoustic power. With the increase of acoustic power, the increased PS levels were observed.Figure 4 shows the box-whisker plot of both PS and τb in the sonicated ROI. The median value of τb shows the decrease in the mean water residence time with the increased level of BBB disruption. In Figure 5, the exchange rates for both Gadolinium and the water (PSw = 1/τb) are compared. As shown in the plot, both exchange rates are increased with the higher acoustic power, while the PSw appears to show more monotonical increase with the acoustic power.
Discussion & Conclusion
The preliminary results of the study suggest that both PS and PSw are sensitive to subtle changes of BBB induced by FUS. Note that the level of PS in the sonicated area is higher than that of the normal brain, but substantially lower than that of tumor (in about 3 orders of magnitude). PS and PSw measures BBB permeability using Gd and water molecules that are noticeably different in their size. Hence, we expect that using these two measures jointly would provide a better picture of BBB changes occurring in various neurodegenerative diseases. The synergistic role of these two measures will be further investigated in future studies.Acknowledgements
NIH R01CA160620References
1. Montagne, A., et al., Blood-brain barrier breakdown in the aging human hippocampus. Neuron, 2015. 85(2): p. 296-302.
2. Heye, A.K., et al., Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage: Clinical, 2014. 6: p. 262-274.
3. Bashir, M.R., et al., Emerging applications for ferumoxytol as a contrast agent in MRI. Journal of Magnetic Resonance Imaging, 2015. 41(4): p. 884-898.
4. Hamilton, B.E., et al., Ferumoxytol-enhanced MRI is not inferior to gadolinium-enhanced MRI in detecting intracranial metastatic disease and metastasis size. American Journal of Roentgenology, 2020. 215(6): p. 1436-1442.
5. Vlachos, F., Y.S. Tung, and E. Konofagou, Permeability dependence study of the focused ultrasound‐induced blood–brain barrier opening at distinct pressures and microbubble diameters using DCE‐MRI. Magnetic resonance in medicine, 2011. 66(3): p. 821-830.
6. Zhang, J. and S.G. Kim, Estimation of cellular‐interstitial water exchange in dynamic contrast enhanced MRI using two flip angles. NMR in Biomedicine, 2019. 32(11): p. e4135.
7. Patlak, C.S., R.G. Blasberg, and J.D. Fenstermacher, Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow & Metabolism, 1983. 3(1): p. 1-7.
8. Tofts, P.S., et al., Estimating kinetic parameters from dynamic contrast‐enhanced T1‐weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 1999. 10(3): p. 223-232.
Figures