2017
Integrative assessment of the aquaporin-4 role to a glucose stimulus by magnetic resonance1Instituto de Investigaciones Biomédicas Alberto Sols, Madrid, Spain
Synopsis
Aquaporin-4 (AQP4) is a transmembrane water channel highly expressed in central nervous system, regulating fluid exchange by water transport between two sides of plasmatic membrane, and depending on concentration gradients of solutes, like glucose. On these grounds, we studied AQP4’s role in glucose uptake. AQP4 inhibitor TGN-020 was administrated in adult mice before vehicle and glucose stimulus. Diffusion and T2* images were acquired and apparent diffusion coefficient and T2* were measured. Ex vivo 1H high-resolution magic angle spinning spectra were acquired. Results showed that TGN avoid physiological cerebral response linked to glucose uptake, noting the AQP4 involvement in the process.
Introduction and objectives
Water homeostasis in the central nervous system (CNS) is of pivotal physiological and clinical importance, since about 80% of the brain’s weight is water. Aquaporin-4 (AQP4) is a water-channel protein mainly expressed in astrocytes end‐feet bordering capillaries. These cells have different functions like maintaining the extracellular ion and water balance1,2. Glucose uptake in CNS is known to elicits water transport, which is linked to a mild cellular swelling effect and microvasculature changes3. On this sense, AQP4 is the main osmoreceptor and regulator of water balance and flow in cerebral tissues1. We have previously shown that functional magnetic resonance imaging (fMRI) can detect these changes associated to glucose uptake4. On these grounds, AQP4 role in these processes was studied.Materials and methods
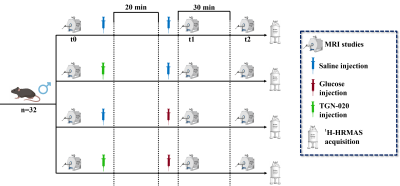
Experimental design: Healthy C57BL6/J adult male mice (n = 32) were fed with a standard laboratory diet and randomly distributed in 4 groups (n= 8 each), where: i) saline group, in which animals were injected with saline (100 μL + 200 μL/25g body weight (b.w.). in two injections); ii) glucose group, with mice injected with saline (100 μL/25g b.w.) plus glucose (200 μL/25g b.w. of 2.08 M glucose in saline); iii) TGN+saline group, including animals injected with the AQP4 inhibitor (100 μL/25g b.w. of 0.24 M TGN-020) plus saline (200 μL/25g b.w.) and iv) TGN+glucose group, which included animals injected with the AQP4 inhibitor (100 μL/25g b.w. of 0.24 M TGN-020) and glucose (200 μL/25g b.w. of 2.08 M glucose in saline). MRI acquisitions sets, and substances administrations were performed (figure 1): Basal measurements (at time t0) were acquired prior to compound administration. Right after, TGN or saline were supplied intraperitoneally (i.p.) through a catheter. 20 minutes after TGN/saline administration, the glucose/saline solution were administrated, and the second set of studies was acquired right after (t1). 30 minutes after glucose or saline injection, a third set of studies was performed (t2). MRI studies: MRI studies were acquired in a 7T equipment. Diffusion and T2* parametric maps were computed with homemade software developed in Matlab. ROIs in hippocampus, hypothalamus and cortex were manually selected and analyzed. HRMAS studies: Animals were euthanized under anaesthesia effect by cervical dislocation. Brain was excised from the skull and different cerebral regions were segmented. The samples collected (cortex, hippocampus, and hypothalamus) were stored separately in small tubes and preserved at -80° until their analysis in a 11.7 T spectrometer. CPMG sequence was used to acquire the 1H spectra, that were processed with LCModel.Results
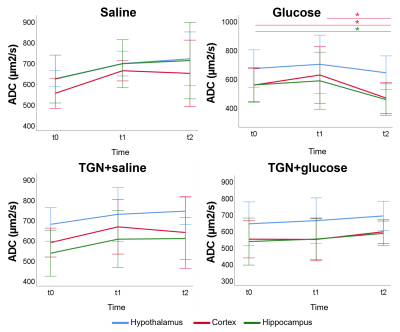
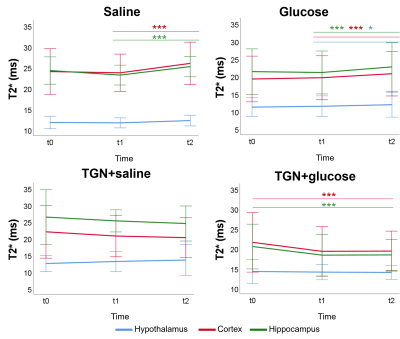
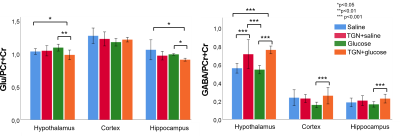
Glucose group presented a decreased ADC 30 minutes after glucose injection in cortex and hippocampus. However, this is inhibited in TGN+glucose group (figure 2). Saline and glucose administration result in an increased T2* 30 minutes after its administrations, in cortex and hippocampus for saline, and in all regions for glucose. These responses are inhibited when TGN is administrated. Moreover, a significant decrease is observed 50 minutes after TGN administration in TGN+glucose group in cortex and hippocampus (figure 3). Metabolomic studies showed a decreased glutamate concentration of TGN+glucose group, as compared to glucose and saline groups, while GABA concentration is higher in both TGN cohorts when compared to saline and glucose groups (figure 4).Discussion
ADC decreases are consistent with glucose-induced neurocellular activity3,5. Under physiological conditions, glucose uptake is linked to mild cellular swelling processes concomitant to the increase in neuronal activity, and changes in water permeability6. Thus, the ADC changes are on agreement with the glucose-induced swelling effects and a consequent decrease of the extracellular space. The water ADC of mice with AQP4 inhibited did not experiment the decrease depicted in the glucose group from t1 to t2, suggesting that the cellular swelling associated to glucose is not taking place. T2* increases after glucose stimulus are on agreement with augmented neuronal activity, which is closely coupled with glucose metabolism and CBF changes7. T2* increases were not observed in either of the TGN cohorts. Furthermore, a specific decrease of T2* was reported. These results indicate that TGN-020 is blocking both the vehicle and glucose-induced T2* changes in the brain. TGN-020 is known to disrupt astrocyte function by blockage of AQP4 channels8, which results in disrupted ion flux of Na+, K+ and Ca+2. Typically, upon neuronal activity through Ca+2 signalling, astrocytes release vasoactive substances (such as glutamate) which promote arteriolar vasodilatation, which is associated with an increasing T2* signal9. Glutamate levels were significantly lower in the TGN+glucose cohort, as compared to the saline and glucose cohorts, in hypothalamus and hippocampus. This is consistent with decreased glucose metabolism, neuronal activity, and water transport. HRMAS data exhibited also significantly higher concentrations of GABA in the TGN+glucose animals than glucose groups in all regions. Since GABA is considered as the main inhibitory neurotransmitter, reducing astrocytes activation would be consistent with decreased T2* values10.Conclussion
Glucose administration induces a cerebral response detectable with diffusion and T2* imaging. TGN inhibits AQP4 and avoids its function, noting that AQP4 is involved in response to glucose uptake. Metabolic results correspond with an alteration of the glutamate cycle due to AQP4 inhibition, that yield in a decreased glutamate and increased GABA.Acknowledgements
No acknowledgement found.References
1-Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543-62.
2-MacAulay N. Molecular mechanisms of brain water transport. Nat Rev Neurosci. 2021;22(6):326-44.
3-van Opstal AM, Hafkemeijer A, van den Berg-Huysmans AA, Hoeksma M, Blonk C, Pijl H, et al. Brain activity and connectivity changes in response to glucose ingestion. Nutr Neurosci. 2020;23(2):110-7.
4-Lizarbe B, Fernández-Pérez A, Caz V, Largo C, Vallejo M, López-Larrubia P, et al. Systemic Glucose Administration Alters Water Diffusion and Microvascular Blood Flow in Mouse Hypothalamic Nuclei – An fMRI Study. Front Neurosci. 2019;13:921.
5-Szabó I, Hormay E, Csetényi B, Nagy B, Lénárd L, Karádi Z. Multiple functional attributes of glucose-monitoring neurons in the medial orbitofrontal (ventrolateral prefrontal) cortex. Neurosci Biobehav Rev. 2018;85:44-53.
6-Tong F, Zou Y, Liang Y, Lei H, Lopsong T, Liu Y, et al. The Water Diffusion of Brain Following Hypoglycemia in Rats - A Study with Diffusion Weighted Imaging and Neuropathologic Analysis. Neuroscience. 2019;409:58-68.
7-Xu F, Liu P, Pascual JM, Xiao G, Huang H, Lu H. Acute effect of glucose on cerebral blood flow, blood oxygenation, and oxidative metabolism. Hum Brain Mapp. 2015;36(2):707-16.
8-Oosuka S, Kida T, Oku H, Horie T, Morishita S, Fukumoto M, et al. Effects of an Aquaporin 4 Inhibitor, TGN-020, on Murine Diabetic Retina. Int J Mol Sci. 2020;21(7):E2324.
9-Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies. Neuroscience & Biobehavioral Reviews. 2014;47:36-52.
10-Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515-7.
Figures