1997
Numerical optimization and validation of Lactate CEST (LATEST) imaging at 3T: In-situ and in-vivo feasibility studies1University Dusseldorf, Medical Faculty, Department of Diagnostic and Interventional Radiology, Düsseldorf, Germany, 2Cent. of Med. Phys. and Biomed. Eng., Friedrich-Alexander-Univ. Erlangen-Nürnberg, Erlangen, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Department of Diagnostic and Interventional Radiology, University Hospital Aachen, Aachen, Germany
Synopsis
Quantification of altered lactate concentrations with non-invasive MR imaging is of diagnostic interest in a broad spectrum of diseases and further analysis of athletic activity. In this context, chemical exchange saturation transfer (CEST) is an increasingly validated contrast mechanism in MRI. However, successful application of CEST requires efficient selective saturation of the exchanging lactate protons. In this study, we successfully optimized and investigated the application of intramuscular lactate CEST imaging (LATEST) supported by numerical simulations and were able to apply LATEST for the first time in-vivo at 3.0 T.
Introduction
Locally altered lactate concentrations are promising biomarkers of a broad spectrum of diseases including cancer, heart failure, and neurologic disorders. In addition, changes in lactate levels also occur because of athletic exertion. Chemical exchange saturation transfer (CEST) is an increasingly validated contrast mechanism that allows indirect visualization of metabolite concentrations1. Often, lactate concentrations oftentimes change by only a few millimoles, so an efficient and selective saturation of exchange protons is required. The exchange processes can be described by the Bloch-McConnell equations. The aims of our study were (1) to optimize a CEST presaturation module for intramuscular imaging of lactate (Lactate Chemical Exchange Saturation Transfer - LATEST) by numerical optimization based on the Bloch-McConnell equations, and (2) to validate the simulation using in-situ and in-vivo measurements.Methods
The exchange processes between the water and the lactate pool were modelled by the Bloch-McConnell equations and analysed using the following parameters: 1) high-frequency field strength (B1), 2) pulse duration (tp), and 3) number of presaturation pulses (np). Thus, the effects of direct water saturation, the size of the magnetization transfer ratio (MTRasym), and the position of the maximum MTRasym were explored. Subsequently, in-situ measurements were performed on nine cadaveric lower leg specimens of deceased body donors (aged 81 ± 9 years) without relevant pre-existent conditions were imaged using a 3T clinical MRI scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) and a dedicated 15-channel knee coil (Tx/Rx Knee 15 Flare Coil, Siemens Healthineers, Erlangen Germany) at room temperature. Following standardized positioning and specimen preparations, baseline CEST measurements (CEST Imaging – WIP 816B, Siemens Healthineers, Erlangen, Germany) using our optimized presaturation parameters were performed. Thereafter, lactate (calcium L-(+)-lactate hydrate, Carl ROTH GmbH & Co. KG, Karlsruhe, Germany) injected intramuscularly into four regions (central in the gastrocnemius muscle), so that lactate concentrations of 0, 5, 20, and 40 mM were present, and CEST measurements were repeated. We also performed in-vivo measurements on the right lower leg of a healthy 26-year-old male. After the baseline CEST measurement, the volunteer performed repetitious plantarflexion and dorsiflexion exercise until exhaustion (all-out exercise), yet prior to any muscle cramps or spasms. During the following 22 minutes, four CEST measurements were acquired to determine time-dependent changes. Z-spectra were frequency-corrected in a pixel-wise manner using WASSR2, and MTRasym values were determined in the lactate-specific range of 0.5-1 ppm3 (LATEST effect). Statistical analyses were performed using Friedman tests and Wilcoxon signed-rank post-hoc tests and the Kendall-Tau b-rank correlation coefficient. Due to the small sample size, the significance level was set at p < 0.01, and the Bonferroni alpha correction was applied.Results
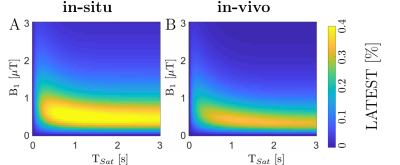
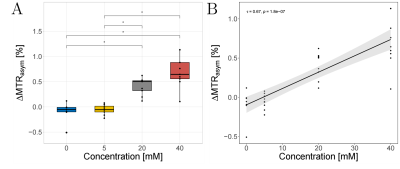
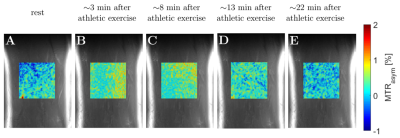
An increase of the LATEST effect (e.g. MTRasym values in the range of 0.5-1 ppm) with saturation time (TSat) was observed for the in-situ and in-vivo simulation parameters (Figure 1). The optimal presaturation parameters were B1 = 0.6 µT, tp = 50 ms, and np = 8 for the in-situ measurements and B1 = 0.5 µT, tp = 50 ms, and np = 15 for the in-vivo measurements. In-situ, we observed significant differences (p < 0.01) in the MRTasym values between the various injected lactate concentrations (Figure 2A) and, correspondingly, a strong and significant (τ = 0.67, p < 0.001) correlation (Figure 2B). In-vivo, a substantial increase of LATEST was observed immediately after exercise. This effect decreased continuously over the following 22 minutes to the baseline value before exercise (Figure 3).Discussion
The results of the in-situ and in-vivo studies showed a lactate concentration-dependent LATEST effect, which was consistent with our simulations. In the in-situ study, significant changes could be detected above a lactate concentration of 5 mM. In the in-vivo study, we observed a substantial increase of the LATEST effect immediately after exercise, which recovered to approximately the resting level during the following 22 minutes, which is in agreement with the previous studies4,5.Conclusion
In this feasibility study, changes in lactate concentration of a few millimoles were successfully detected using an optimized LATEST imaging protocol, providing proof of accurate quantification of altered lactate levels in-vivo with potential future application in a variety of other diseases.Acknowledgements
No acknowledgement found.References
1. Lenich T, Pampel A, Mildner T, Möller HE. A new approach to Z-spectrum acquisition: prospective baseline enhancement (PROBE) for CEST/Nuclear Overhauser Effect. Magn Reson Med. 2019;81(4):2315-2329. doi:10.1002/mrm.27555 .
2. Kim M, Gillen J, Landman BA, Zhou J, van Zijl PCM. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441-1450. doi:10.1002/mrm.21873 .
3. Saito S, Takahashi Y, Ohki A, Shintani Y, Higuchi T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) at 7T-MRI. Radiol Phys Technol. 2019;12(1):46-54. doi:10.1007/s12194-018-0490-1 .
4. DeBrosse C, Nanga RPR, Bagga P, et al. Lactate Chemical Exchange Saturation Transfer (LATEST) Imaging in vivo A Biomarker for LDH Activity. Sci Rep. 2016;6(1):19517. doi:10.1038/srep19517 .
5. Gaitanos GC, Williams C, Boobis LH, Brooks S. Human muscle metabolism during intermittent maximal exercise. Journal of Applied Physiology. 1993;75(2):712-719. doi:10.1152/jappl.1993.75.2.712 .
Figures