1995
Detection of low-boiling point perfluorocarbon nanodroplets using hyperCEST: a path toward a dual-modality dual-phase contrast agent1Physics & Astronomy, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3Pharmacoengineering and Molecular Pharmaceutics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Recently, it was shown that hyperCEST enables detection of microbubbles at clinically relevant ultrasound doses. Nanodroplets filled with low-boiling point perfluorocarbons are precursors of microbubbles. Because the chemical shift of xenon in liquid-phase perfluorocarbon nanodroplets is different than that in gas-phase microbubbles, hyperCEST detection of these nanodroplets could enable MR detection of their phase-change upon ultrasound activation. To this end, here we investigate if perfluorocarbon nanodroplets can be used as hyperCEST agent, first in vitro and then in vivo.
Introduction
Perfluorocarbon nanodroplets are composed of synthetic molecules with high gas-solubility, and they have been used for transport and delivery of gases for therapeutic purposes1,2. Xenon is highly soluble in perfluorocarbons3, and nanodroplets may provide a means for intravenous delivery of hyperpolarized (HP) 129Xe for in vivo MR applications4. Nanodroplets filled with low-boiling point perfluorocarbons are a new class of ultrasound contrast agents (dual-phase contrast) that can be easily converted into gas microbubbles when exposed to low-pressure ultrasound waves1. While MR detection of microbubbles requires doses that far exceed those used in clinical ultrasound imaging, it was recently demonstrated that hyperCEST enables the detection of microbubbles at clinical in-blood concentrations5. The scope of this work was to assess if hyperCEST also enables the detection of low-boiling point perfluorocarbon nanodroplets, which ultimately may enable their use as phase-change contrast agent for both ultrasound and MRI.Methods
Nanodroplets were prepared via the condensation method as described in Sheeran et al6. Briefly, a solution of DSPC and DSPE-PEG2000 (Avanti Polar Lipids, Alabaster, AL, USA) at a 9:1 molar ratio in phosphate buffered saline, propylene glycol, and glycerol (16:3:1) was prepared. The lipid solution was degassed under vacuum, and the vial headspace was replaced with decafluorobutane gas (Fluoromed, Round Rock, TX, USA). Bubbles were generated via mechanical agitation. Nanodroplets were condensed by cooling the bubbles to -13 °C in a chilled ethanol bath, followed by vial pressurization with compressed nitrogen at 20 PSI. Droplets were prepared at original concentration (OC, 1010 droplets/mL), high concentration (HC, 1011 droplets/mL), and low concentration (LC, 109 droplets/mL).All in vitro spectroscopic studies were made on a high resolution 500 MHz NMR spectrometer (Varian NMR Systems, Palo Alto, California, USA). The droplets were equilibrated to 37 °C to replicate in vivo conditions. Fresh HP 129Xe was periodically bubbled into the sample, followed by presaturation with a 90° continuous wave excitation pulse (B1 = 3 μT applied for 2 s), and signal acquisition. This was repeated across a spectral width, and the resulting Z-spectrum was used to identify the chemical shift of 129Xe within the droplets.
In vivo spectroscopic studies were performed using a 9.4 T small animal MR scanner (BioSpec 94/30, Bruker Biospin, Billerica, MA) using a 35 mm dual-tuned 1H/129Xe volume coil (m2m Imaging Corp., Cleveland, OH). A HC droplet solution was injected into a mouse using a tail vein catheter to reach an in-blood concentration similar to the LC used for in vitro experiments. For these experiments, the mouse was mechanically ventilated with a mixture of HP 129Xe/N2. In vivo hyperCEST was done as previously described using optimized triggering between the ventilation cycle, pulsed-presaturation (B1 = 10 μT applied for 320 ms), and signal acquisition7.
Results
Figure 1 shows in vitro results. With OC, the 129Xe signal within the droplets were observed directly at -139 ppm with respect to the dissolved-phase signal (Figure 1A inset). When the concentration was lowered to LC, the droplets could no longer be observed directly. However, presaturation (B1 = 3 μT applied for 2 s) at -139 ppm resulted in significant dissolved-phase signal attenuation (Figure 1B). Figure 2 shows in vivo results. Like in vitro, the droplet signal cannot be directly detected (Figure 2A). The in vivo hyperCEST Z-spectrum shows a broad valley centered at -194 ppm that corresponds to the direct saturation of the gas-phase magnetization within the lungs of the mouse (Figure 2B). The width of this valley is too broad to enable clear identification of any hyperCEST effect from the nanodroplets.Discussion
Our in vitro data clearly show that perfluorocarbon nanodroplets can be used as hyperCEST agent at the concentration typically used for ultrasound studies. Their in vivo detection, however, presents several challenges compared to other hyperCEST agents like cucurbit[6]uril7. The chemical shift of these nanodroplets is too close to the gas-phase signal originating from the lungs. A formal magnetization transfer ratio asymmetry analysis8 centered around the gas-phase signal is required for their detection. The broadening of the valleys between the in vitro and in vivo Z-spectra is due to the necessary differences in presaturation pulse shapes. It is important to note that previous HP 129Xe MR studies have reported in vivo detection of preloaded nanodroplets at high concentrations4,9. Also, when ventilating with HP 129Xe gas, Venkatesh et al. could not directly resolve a nanodroplet signal, even after replacing half of the blood volume with the emulsion. Further studies are warranted to assess whether the large gas-phase peak is obscuring the hyperCEST effect of the nanodroplets, or if chemical exchange between the droplets and blood is severely impeded.Conclusion
Nanodroplets filled with low-boiling point perfluorocarbons are a dual-phase contrast agent that can easily be converted into microbubbles using ultrasound. Their detection by hyperCEST, at the clinically relevant doses used for ultrasound, could enable their use as dual-phase dual-modality contrast agent. Here, we show that these nanodroplets display a strong hyperCEST effect in vitro, but their in vivo detection is either obscured by direct saturation of the large HP 129Xe gas reservoir in the lungs, or is prevented by less favorable exchange dynamics in the blood, warranting further studies.Acknowledgements
This work was supported by the National Institute of Biomedical Imaging and Bioengineering trough grant number R21EB031319, and by the National Institutes of Diabetes and Digestive and Kidney Diseases trough grant numbers R01DK108231 and R01DK12306.
References
1. Durham PG, Dayton PA. Applications of sub-micron low-boiling point phase change contrast agents for ultrasound imaging and therapy. Curr Opin Colloid Interface Sci. 2021;56:101498. doi:10.1016/J.COCIS.2021.101498
2. Jägers J, Wrobeln A, Ferenz KB. Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflügers Arch - Eur J Physiol 2020 4732. 2020;473(2):139-150. doi:10.1007/S00424-020-02482-2
3. Pollack GL, Kennan RP, Holm GT. Solubility of Inert Gases in PFC Blood Substitute, Blood Plasma, and Mixtures. https://doi.org/103109/10731199209119768. 2009;20(4--Feb):1101-1104. doi:10.3109/10731199209119768
4. Wolber J, Rowland IJ, Leach MO, Bifone A. Perfluorocarbon emulsions as intravenous delivery media for hyperpolarized xenon. Magn Reson Med. 1999;41(3):442-449. doi:10.1002/(SICI)1522-2594(199903)41:3<442::AID-MRM3>3.0.CO;2-7
5. McHugh CT, Durham PG, Kelley M, Dayton PA, Branca RT. Magnetic Resonance Detection of Gas Microbubbles via HyperCEST: A Path Toward Dual Modality Contrast Agent. ChemPhysChem. 2021;22(12):1219-1228. doi:10.1002/CPHC.202100183
6. Sheeran PS, Rojas JD, Puett C, Hjelmquist J, Arena CB, Dayton PA. Contrast-Enhanced Ultrasound Imaging and in Vivo Circulatory Kinetics with Low-Boiling-Point Nanoscale Phase-Change Perfluorocarbon Agents. Ultrasound Med Biol. 2015;41(3):814-831. doi:10.1016/J.ULTRASMEDBIO.2014.10.020
7. McHugh CT, Kelley M, Bryden NJ, Branca RT. In vivo hyperCEST imaging: Experimental considerations for a reliable contrast. Magn Reson Med. October 2021. doi:10.1002/MRM.29032
8. van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65(4):927-948. doi:10.1002/mrm.22761
9. Venkatesh AK, Zhao L, Balamore D, Jolesz FA, Albert MS. Evaluation of career agents for hyperpolarized xenon MRI. NMR Biomed. 2000. doi:10.1002/1099-1492(200006)13:4<245::AID-NBM635>3.0.CO;2-7
Figures
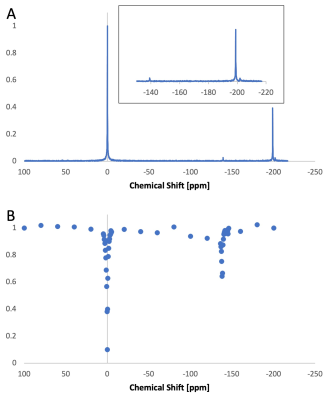
Figure 1. In vitro hyperCEST with low-boiling point perfluorocarbon nanodroplets. (A) HP 129Xe spectrum from the HC nanodroplet suspension. At HC, the signal from 129Xe dissolved in the nanodroplets can be directly detected -139 ppm (inset). A large gas-phase signal is observed at -198 ppm originating from the peek tubing used to delivered the HP gas, while the peak at -202 ppm corresponds to microbubbles formed from droplet vaporization. (B) Direct saturation of xenon within the liquid nanodroplets produced a 35% reduction of the dissolved-phase signal, here placed at 0 ppm.
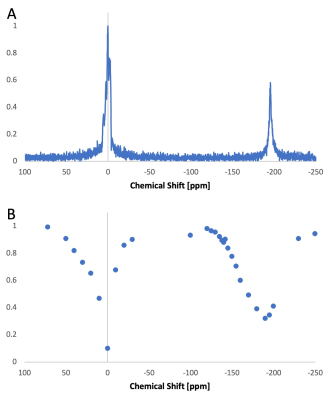
Figure 2. In vivo detection of low-boiling point perfluorocarbon nanodroplets. (A) In vivo HP 129Xe spectrum showing a large dissolved-phase signal (0 ppm), originating from xenon dissolved in blood and tissue, and a gas phase signal (-194 ppm), originating from HP 129Xe in the lungs; (B) In vivo Z-spectrum only shows a broad valley centered at -194 ppm.