1994
HyperCEST Performance of Liposomal Nanocarriers using a CrA-Lipopeptide Building Block1Translational Molecular Imaging, Deutsches Krebsforschungszentrum, Heidelberg, Germany
Synopsis
MRI reporters for hyperpolarized 129Xe CEST need further improvement in order to sufficiently outcompete the intrinsic loss of hyperpolarization appearing under in vivo conditions. This study opens up a possibility by accelerating the CEST build-up for the well-known Xe host CrA-ma by two orders of magnitude. We designed a liposome decorated with a CrA-lipopeptide. To avoid inefficiency from back exchange, the lipopeptide fraction should be kept relatively low (e.g., 2 mol%). Complete saturation transfer could be easily achieved for maximum possible image contrast in 129Xe MRI scans. This study pinpoints a large flexibility for designing powerful HyperCEST agents.
Introduction
HyperCEST MRI with xenon biosensors combines the signal enhancements provided by hyperpolarized 129Xe and by chemical exchange saturation transfer. It reaches unprecedented high sensitivity for in vitro studies1 but still lacks the translation to meaningful in vivo experiments. One reason for this lies in the relative elaborated synthesis to join Xe hosts to a targeting unit and another in the significantly enhanced relaxation of the hyperpolarization under physiological conditions. The original design approach for Xe biosensors needs to be revisited with regard to faster Xe exchange, flexible synthesis/assembly routes, and “multivalent” sensors for more than one Xe atom. Previous liposomal approaches2 suffer from “bleeding” of the Xe host and poor control over the liposome loading.Methods
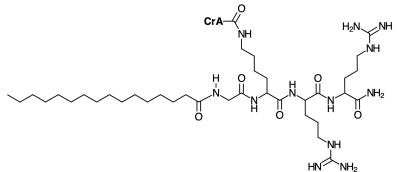
To this end, we have designed a lipopeptide for insertion into POPC liposomes. The peptide section carries cryptophane-A mono-acid (CrA-ma) as a well-studied Xe host. CrA-ma has been coupled to a Lys side chain (see Fig. 1) at a position where the hydrophobic host can insert into the phospholipid bilayer. The lipopeptide serves as a modular building block and enables controlled loading of nanocarriers (NCs) with Xe hosts and their persistent inclusion into the lipid environment with favorable exchange conditions. We investigated the HyperCEST performance of different CrA-lipopeptide loads and compared it to that of free CrA-ma. This work also discusses the challenges for quantitative comparison of HyperCEST agents with rather different performance. This issue arises when the observed CEST signature becomes insensitive to changes in the saturation conditions. A stepwise comparison via the depolarization time is proposed to compare the signal build-up with increasing CEST preparation. Xe MRI and CEST spectroscopy was performed on a 9.4 T preclinical system with a home-build polarizer for spin exchange optical pumping and precise Xe dissolution delivery.Results
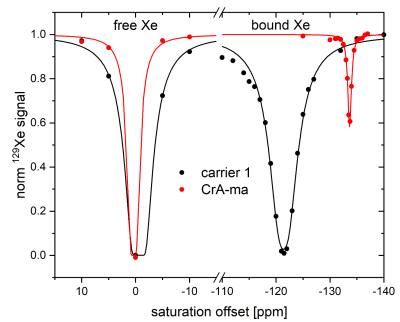
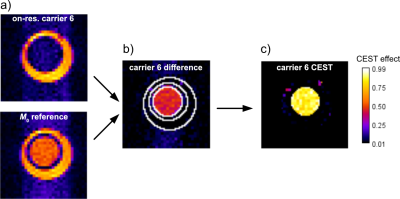
Liposomes with different formulations regarding lipopeptide load (20 mol% vs. 2 mol%), cholesterol content (0 vs. 10 mol%), and PEGylation with PE-PEG2000 were generated and showed stable diameters of 105-125 nm. A solution of carrier 1 with 20 mol% lipopeptide at 10 µM cage equivalent showed 100% CEST effect in a solution bubbled with 2% Xe (90 mbar) with a saturation of 20 s with 1 mW (see Fig. 2). This is an impressive effect given that the response from unfunctionalized CrA-ma only yields ca. 40% CEST effect at equivalent concentration. The quantitative evaluation of the z-spectra in direct comparison is partially masked by the excessive response from the liposomes.This prompted us to quantify the improvement of carriers with 20 and 2 mol% lipopeptide compared to CrA-ma based on the depolarization time τ for an increasing saturation time and a fixed saturation power (1 mW). The NC with 20 mol% lipopeptide (τ = 3.65±0.09 s) was ca. 17-fold more efficient than CrA-ma at equivalent cage concentration (τ = 62±1 s). A lower cage load (2 mol%) yielded further improvement by another factor of 5 compared to the 20 mol% formulation. PEGylation and insertion of 10 mol% cholesterol did not have a negative impact on the observed saturation responses and can thus be used for tuning the membrane conditions. Overall, the carriers with low cage load performed ca. 100-fold better than unfunctionalized CrA-ma. One type of NC with 2 mol% lipopeptide was chosen for MRI experiments and yielded a strong CEST response at 2 µM cage equivalent (see Fig. 3). The same amount of free CrA-ma did not show any image contrast when saturation was applied to the respective frequency at ca. 12 ppm upfield shift from the lipopeptide response.Discussion and conclusion
Liposomes loaded with lipopeptide-anchored CrA as Xe host provide excellent HyperCEST contrast. This first generation of the proposed carriers already provides an improvement of ca. 2 orders of magnitude. The efficiency of CrA with medium Xe exchange kinetics in aqueous environment is thus significantly enhanced and the hydrophobic Xe host in this newly designed microenvironment delivers faster turnover of the noble gas. Direct comparison in z-spectra with full saturation from liposomes and weak saturation from the individual hosts is challenging as the quantitative analysis can be ambiguous. Alternatively, the depolarization time τ is a suitable quantitative measure to rank the performance of different formulations. Further tuning of the cage load and of the membrane conditions might lead to additional improvements. A liposome loading with only 2 mol% CrA-lipopeptide yields better results than the 20 mol% formulation, presumably due to the lack of back exchange when the CrA load is kept low. Overall, this study illustrates the wide parameter space that is now available when incorporating CrA-labelled lipopeptides into liposomal carriers. The functionalization of such nanocarriers will be investigated in an upcoming study.Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Koselleck Grant No. 316693477 (SCHR 995/5–1). Support by the Dieter Morszeck Stiftung is also gratefully acknowledged.References
1. Jayapaul, J.; Schröder, L. Molecular Sensing with Host Systems for Hyperpolarized 129Xe. Molecules 2020, 25 (20), 4627.
2. Schnurr, M.; Volk, I.; Nikolenko, H.; Winkler, L.; Dathe, M.; Schröder, L. Functionalized Lipopeptide Micelles as Highly Efficient NMR Depolarization Seed Points for Targeted Cell Labelling in Xenon MRI. Adv. Biosyst. 2020, 4 (3), 1900251.
Figures