1992
Highly sensitive “off/on” EPR probes to monitor enzymatic activity by OMRI
Simonetta Geninatti Crich1, Sabrina Geninatti Elkhanoufi2, Diego Geninatti Alberti2, Eric Thiaudiere3, Rachele Stefania1, Elodie Parzy3, Philippe Mellet3, Philippe Massot3, and Silvio Aime1
1University of Torino, Torino, Italy, 2University of torino, Torino, Italy, 3University of Bordeaux-CNRS, Bordeaux, France
1University of Torino, Torino, Italy, 2University of torino, Torino, Italy, 3University of Bordeaux-CNRS, Bordeaux, France
Synopsis
New stable organic radicals probes have been proposed in this study to perform Molecular Imaging by monitoring by Overhauser Magnetic Resonance Imaging (OMRI) the enzymatic activity. The radicals used are Tempo-containing esters forming stable micelles that are practically EPR silent. The hydrolysis of the ester bond catalyzed by Carboxyl esterases generates a narrow and intense EPR signal as a consequence of the release of the nitroxide radical from the micelle. Thus we have prepared a off/on probe responsive to the carboxylesterase activity that can be detected quantitatively in the OMR image.
Introduction
Despite the effort done in the last 20 years, molecular imaging by MRI remains a challenge. One of the reasons is the gap that occurs between the low biomarkers concentration and the minimum Gd contrast agent concentration needed to detect a specific contrast. Overhauser MRI and PEDRI approaches[1], that are double resonance experiments based on the magnetization transfer from the high electron magnetic moment (on a stable radical) to the water proton located close to this radicals obtained by the saturation of the radical electronic transitions, are considered an interesting alternative approach to map enzymatic activity “in vivo”. In fact, the transfer of a part of the high spin polarization of an unpaired electron of an organic radical enhances the MRI signal that appears brighter, that become reporter about the stable radicals biodistribution. To this purpose, suitably functionalized stable organic radicals (TEMPO-like derivatives) may behave as substrates for specific enzymes over-expressed in pathological tissues, thus allowing to design efficient molecular imaging tests. In this study, we investigated the use of “off/on” nanoparticles taking advantage of the fact that nitroxide radicals are “quenched” when they are immobilized on nanostructures like micelles or liposomes. Thus in intact micelles or liposomes the radicals are almost EPR silent showing a low and broad EPR signal. When the radical is linked to the immobilizing aliphatic chain by an ester bond, the hydrolysis of this linkage catalyzed by esterases generates a narrow and intense EPR signals as the result of the release of the nitroxide radicals from the nanoparticles. Both these enzymes are an important target in cancer diagnosis and in the assessment of applied therapies.Methods
TEMPO derivative nitroxide radicals were conjugated to a fatty acid (dodecanoic acid) via the formation of an ester bond to yield Tempo-C12 (TC12) and Tempo-2-C12 (T2C12) (figure 1). The esterification reaction of Tempo derivatives with lauroyl chloride was carried out in a one-step reaction[2] using pyridine as a base and in a two-step3 process, through enolate formation, using lithium diisopropyl amide (LDA) as a strong base to obtain compound T-C12 and T-2-C12, respectively. The EPR spectra were acquired with Adani EPR spectrometer Spinscan X (9.2-9.55 GHz). The OMRI system used in all experiments is an EPR cavity (Bruker, Wissemburg, France) inserted at the center of Cirrus Open 0.2 T MRI system (MRI Tech, Canada). This permanent magnet at 0.193 T is operated at a proton frequency of 8.24 MHz and maximal field gradient strength was 20 mT/m in the three directions of space. The EPR cavity operates at 5.44 GHz[3]Results
Both Tempo-containing esters (TC12 and T2C12) exhibit a low solubility in water and aggregate to form stable micelles with the lipophilic tail in the core and the nitroxide radical exposed to water. The radicals in the micellar aggregates are practically EPR silent showing a low and broad EPR signal. The hydrolysis of the ester bond catalyzed by Carboxylesterase 1 and 2 generates a narrow and intense EPR signal as a consequence of the release of the nitroxide radical from the micelle, that is proportional to the enzymatic activity. Other hydrolases and proteins such as HSA, BSA, Human Serum, Lysozyme, Trypsin, Protease and Phospholipase A2 were also investigated without observing any detectable hydrolyzing capacity on the two compounds considered in this work The enzymatic activity was well detected in the corresponding OMRI images acquired at 0.193T (Figure 2)conclusions
In summary, the method has the potential to be translated to in vivo applications via OMRI (Overhauser Magnetic Resonance Imaging) protocols. For this application, the use of probes characterized by narrow EPR signals is crucial to provide high sensitivity and specificity in the polarization transfer from electrons to water protons. The “off-on” transition makes the methodology extremely sensitive and quantitative avoiding the use of complex ratiometric corrections to eliminate the contribution arising from the not hydrolyzed probes. In fact, the sensitivity observed in an in vitro experiment for carboxylesterase 2 using T2C12 as substrate was ( 8.00 x 10-5 U/mL), i.e. of the same order of the values achieved with optical methods. The availability of the herein described nanosized probes may contribute to the in vivo OMRI detection of specific enzymatic activities.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 86309, Primogaia project.References
[1]Lurie DJ, Bussell DM, Bell LH, Mallard JR. Proton electron double magnetic-resonance imaging of free-radical solutions. J Magn Reson 1988; 76: 366– 370. [2]C. Aliaga, F. Bravo-Moraga, D. Gonzalez-Nilo, S. Márquez, S. Lühr, G. Mena and M. C. Rezende, Food Chem, 2016, 192, 395–401. [3RivotA., Jugniot N., Jacoutot S., Vanthuyne N., Massot, P., Mellet P., Marque S.R.A, Audran G., Voisin P., Delles M., Devouassoux G., Thiaudiere E., Bentaher A., Parzy E. Magnetic Resonance Imaging of Protease-Mediated Lung Tissue Inflammation and Injury. ACS Omega 6:23 (2021) 15012–15016.Figures
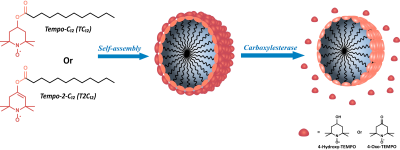
Figure 1 Chemical
structure of Tempo-C12 (T-C12) and Tempo-2C12 (T-2C12); graphic representation
of the micelles obtained with the two compounds and release of the radical in
presence of the CE.
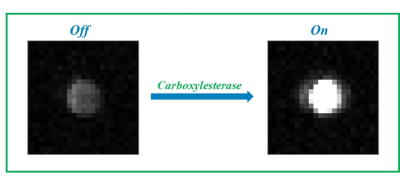
Figure 2 OMR Image
obtained after the incubation of T2C12 (1 mM) for 4 hours in the absence (left)
and in the presence of carboxyl esterase 2 (500 nM)
DOI: https://doi.org/10.58530/2022/1992