1990
A Novel Manganese EOB-Pyclen Diacetate Chelate for Liver-Specific MRI1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States
Synopsis
MRI is increasingly utilized for the diagnosis of liver disease and focal liver lesions. While liver-targeted gadolinium-based contrast agents (GBCAs) have high efficacy, they are shadowed by safety concerns regarding tissue retention. We have developed a liver-targeted manganese alternative – Mn-EOB-PC2A – that uses a liver-targeted ethoxybenzyl modified macrocyclic pyclen diacetate platform. Relaxivity measurements for Mn-EOB-PC2A suggest comparable performance to the GBCA alternatives, and in vitro characterization suggests strong uptake in hepatocytes with minimal toxicity. MRI with Mn-EOB-PC2A demonstrated strong liver-specific enhancement at a clinically relevant dose, underscoring the potential for Mn-EOB-PC2A as an alternative to traditional liver-targeted Gd-based platforms.
Introduction
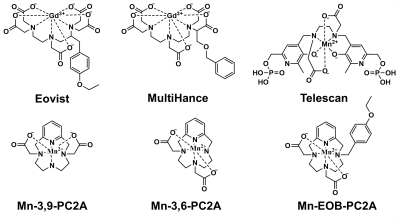
Magnetic resonance imaging (MRI) is commonly utilized for diagnosing liver diseases and focal liver lesions1. While direct biopsy of the affected liver tissue is the current reference standard, the invasiveness of the procedure and its ability to sample just a small portion of the liver limit its application2. Imaging techniques like MRI offer a noninvasive tool for analyzing entire organ systems in a single procedure, allowing visualization of minute details within organs for more accurate diagnosis.Currently, the only clinically used contrast agents are gadolinium (Gd)-based contrast agents (GBCAs). Those that are specific to the liver – Eovist and MultiHance – utilize hydrophobic ligands targeted to the OATP1 transporter on the surface of hepatocytes (Fig 1)3. Specifically, the ligand in Eovist – ethoxibenzyl (EOB) – facilitates upwards of 50% uptake of the injected dose into the liver, whereas other agents see >95% excretion via renal filtration4. However, these agents use a linear chelate structure, which has significantly lower chelate stability than macrocyclic structures, and the administration of GBCAs is known to trigger nephrogenic systemic fibrosis in patients with damaged or failing kidneys5,6.
Manganese (Mn), on the other hand, has strong intrinsic paramagnetic properties and labile water exchange, and is an endogenous ion in the body7. While Mn-based contrast agents (MBCAs) have experienced clinical success in the form of Telescan, Telescan uses a less stable linear chelate and has low relaxivity relative to its Gd-based counterparts (Fig 1)7. Recently, a promising new class of macrocyclic chelating ligands for Mn2+ – pyclen diacetate (PC2A) – has been developed offering high stability and high relaxivity (Fig 1)8. This research aims to apply the liver-targeting EOB ligand from Eovist to the pyclen diacetate platform to develop a novel liver-targeted MBCA comparable in performance to clinically-used GBCAs.
Methods
After synthesis and purification of the novel liver-targeted MBCA – Mn-EOB-PC2A – solutions of varying concentrations of Mn-EOB-PC2A were prepared in DPBS or saline containing 4.5% human serum albumin. The relaxation rates of the phantoms were measured at 1.5T using a Bruker Minispec Reloxometer, and a linear curve was fit to the data to calculate relaxivity. The hepatoma cell line, HepG2, which is commonly used as a proxy for primary liver cells, were cultured in the presence of varying concentrations of Mn-EOB-PC2A for 48 hours, and cell viability was determined via CCK8 assay. To determine the uptake of Mn-EOB-PC2A, HepG2 cells cultured in 2 mM Mn-EOB-PC2A were dissolved and underwent inductively-coupled plasma optical emission spectroscopy (ICP-OES) to calculate the intracellular Mn concentration. To assess the efficacy of Mn-EOB-PC2A for liver MRI, C57BL6 mice were administered 0.060 mmol/kg Mn-EOB-PC2A and imaged before and at 30 minutes after injection with T1-weighted 3D FLASH sequences to determine the distribution of the agent. Signal-to-noise ratio (SNR) was calculated for various tissues for comparison based on the coronal slices from the 3D FLASH acquisition. Maximum intensity projections (MIPs) were produced to visualize the distribution of enhancement in the mice.Results and Discussion
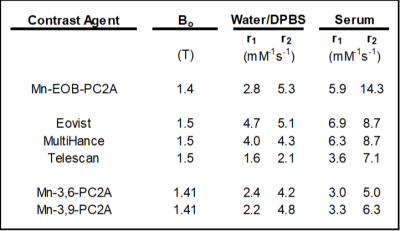
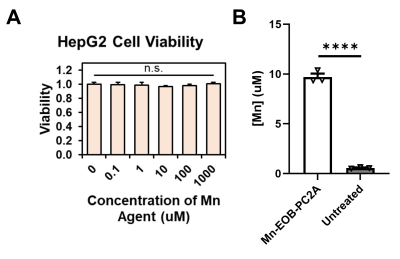
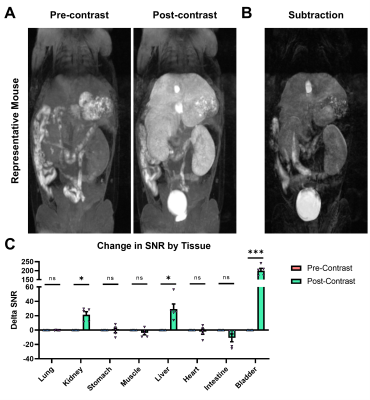
In DPBS, the r1 and r2 relaxivities of Mn-EOB-PC2A are 2.8 and 5.3 mM-1s-1, respectively, both of which are slightly higher but comparable to the other PC2A chelates (Fig 2)8. In 4.5% human serum albumin, the r1 and r2 relaxivities of Mn-EOB-PC2A increase to 5.9 and 14.3 mM-1s-1, respectively, which represents a stark increase over the other PC2A chelates and is comparable to the relaxivities of both Eovist and MultiHance (Fig 2)8,9. In all cases, Mn-EOB-PC2A exhibited substantially higher relaxivity than Telescan (Fig 2)9. Cell viability analysis determined no cellular toxicity of Mn-EOB-PC2A, suggesting a good safety profile at the cellular level (Fig 3A). ICP-OES analysis revealed a 17-times increase in the intracellular Mn concentration after incubation with Mn-EOB-PC2A relative to untreated controls (Fig 3B). While the increased intracellular Mn is substantially lower than the incubation concentration, hepatocytes are known to utilize MDR1 transporters to expel excess agent from the cell3,4.MRI of C57BL6 mice after Mn-EOB-PC2A administration gave robust signal enhancement throughout the liver, kidneys, and bladder, as suggested by the 3D FLASH MIPs (Fig 4A). Eovist, the clinically used EOB-targeted GBCA, exhibits roughly 50% uptake into the liver and 50% excretion via renal filtration, which is consistent with the enhancement distribution of Mn-EOB-PC2A4. The strong gallbladder enhancement in the superior liver, prominent in the subtraction MIP, suggests elimination of Mn-EOB-PC2A via the biliary tree, supporting the strong uptake of Mn-EOB-PC2A into, and eventual expulsion from, hepatocytes (Fig 4B). Importantly, no other off target tissues, including the heart and lungs, exhibit any enhancement related to administration of Mn-EOB-PC2A, as suggested by the SNR analysis of various tissues (Fig 4C). This data demonstrates that Mn-EOB-PC2A offers robust enhancement in the liver at applicable doses for clinical use, expanding the PC2A platform for clinical translation.
Conclusion
Mn-EOB-PC2A represents a promising derivative of the macrocyclic Mn-PC2A chelate platform. We have demonstrated the strong uptake and enhancement of Mn-EOB-PC2A in the liver, as well as the safety of Mn-EOB-PC2A at both the cellular and systemic levels in immunocompetent mice. Our work demonstrates the potential for Mn-EOB-PC2A as a non-Gd alternative to traditional linear GBCAs targeted to the liver at clinically relevant doses without sacrificing enhancement performance.Acknowledgements
This research was supported by the National Institutes of Health grant R01CA211762.References
1. Matos AP, Velloni F, Ramalho M, et al. Focal liver lesions: practical magnetic resonance imaging approach. World J Hepatol. 2015;7(16):1987-2008.
2. Petitclerc L, Sebastiani G, Gilbert G, et al. Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45(5):1276-1295.
3. Francisco FA, de Araujo AL, Oliveira Neto JA, et al. Hepatobiliary contrast agents: differential diagnosis of focal hepatic lesions, pitballs and other indications. Radiol Bras. 2014;47(5);301-309.
4. Seale MK, Catalano OA, Saini S, et al. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29(6):1725-1748.
5. Schmitt-Willich H. Stability of linear and macrocyclic gadolinium based contrast agents. Br J Radiol. 2007;80(955):581-582.
6. Woolen SA, Shankar PR, Gagnier JJ, et al. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent. JAMA Intern Med. 2020;180(2):223-230.
7. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67(44):8431-8444.
8. Garda Z, Molnar E, Hamon N, et al. Complexation of Mn(II) by rigid pyclen diacetates: equilibrium, kinetic, relaxometric, density functional theory, and superoxide dismutase activity studies. Inorg Chem. 2021;60(2):1133-1148.
9. Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40(11):714-724.
Figures