1988
Biosynthesis of an Activatable Fluorescent MRI Contrast Agent1Department of Biomedical Engineering, Michigan State University, East Lansing, MI, United States, 2Division of Synthetic Biology, Institute for Quantitative Health Sciences and Engineering, Michigan State University, East Lansing, MI, United States, 3Department of Radiology, Michigan State University, East Lansing, MI, United States, 4Department of Plant and Microbial Biology, University of California, Berkeley, Berkeley, CA, United States
Synopsis
Sustainable products are becoming an integral part of our daily lives - in this study, we biosynthetically produced an MRI contrast agent from E.coli with an r1 of 6.0 (per-gadolinium, 7T field strength, room temperature) that provides the user with optical feedback through a jump in green fluorescence upon binding free gadolinium in solution. We anticipate this will reduce the amount of resources spent on quality control (in assessing the amount of unchelated gadolinium that could otherwise introduce toxicity), whereas its compatibility with lyophilization is expected to improve storage conditions in terms of longevity, stability, and transportation costs.
Objective
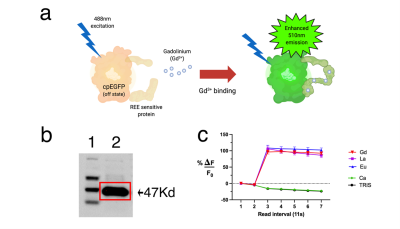
Objective: Studies involving the binding of proteins and rare earth elements (REE) are attractive to many researchers due to the versatility of proteins and unique attributes of rare earths. Among REEs, gadolinium is the most commonly used element for molecular imaging in the clinic with more than 30 million injections a year1. Several protein-based MRI contrast agents have been reported with attributes such as enhanced relaxivity, stability, and targeting capabilities2. Synthesis of these contrast agents can be simply achieved via bioreactors, allowing mass production at an affordable price while eliminating several steps and byproducts from chemical reactions. Here, we sought out to create a biosynthetically producible recombinant protein that directly indicates binding through fluorescence with high specificity for REEs, which can be utilized downstream as an MRI contrast agent upon binding gadolinium. We have developed such a probe and have termed this new construct GLamouR (Green Lanmodulin-based Reporter, Figure 1a).Methods
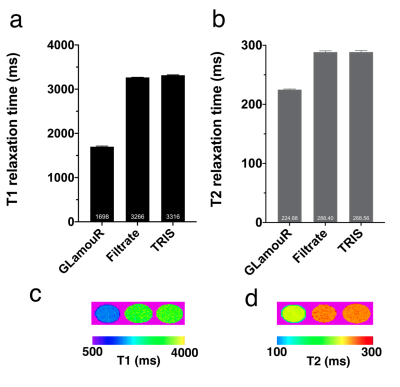
Glamour is a recombinant protein based on modification of the Ca2+ genetically encoded sensor (GCaMP) in which four calcium binding motifs were synonymously replaced with lanthanide binding motifs from the protein Lanmodulin. Protein was expressed in E. coli and isolated with HIS-tag purification and size exclusion. Western blot against the V5 tag (fused to the C terminus of the protein) was used to cross-confirm purification (Figure1b). Samples were dialyzed with TRIS buffer to remove residual imidazole in the solution. Fluorescence was acquired every 11 seconds, and REEs were added after the initial two readings. MRI measurements were performed under a field strength of 7T with samples in 1.5 mL centrifuge tubes. T1 relaxation times were acquired in six 1 mm slices with 12 TR experiments ranging from 800 ms to 17500 ms, averaged 3 times. T2 relaxation times were acquired in the same geometry, with 75 echo images from 7.5 ms to 562.5 ms averaged 7 times. For relaxivity assessment, data was collected from several (n=13 samples) dilutions of six separately purified batches of protein, expressed from bacterial cultures of randomly picked colonies.Results
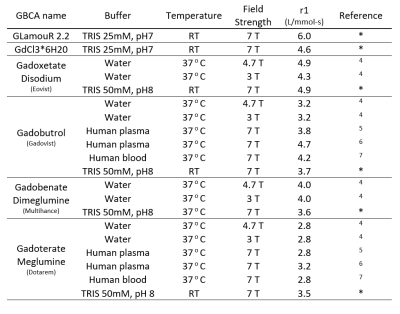
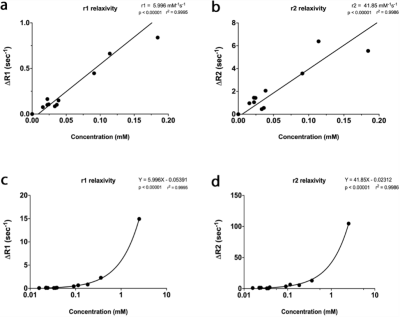
Emission at 510 nm (Figure 1c; n=5 wells with 10 averages, confirmed with separately purified GLamouR from 3 independent colonies) were observed to change +105.2% (±5.6) for Gd, 113% (±9.6) for La, and 116.6% (±7.9) for Eu, when compared to the first read. Negative controls were -10.5% for Ca (±1.6) and -11.2% (±2.0) for TRIS buffer. Decrease in the controls are thought to be attributed to dilution upon adding REEs. MRI data from the pilot study (Figure 2) shows the T1 relaxation time was shortened by approximately 49% from 3316 (± 9) ms for TRIS buffer to 1698 (±16) ms for TRIS solution containing the GLamouR-Gd complex. T2 relaxation time was shortened from 288.1 (±2.7) ms to 224.5 (±1.3) ms, resulting in a decrease of about 22%. The calculated r1 of the GLamouR was 6 L/mmol-s on a per-gadolinium basis as shown in Table 1. Moreover, lyophilized protein that had been reconstituted with distilled, deionized water retained its gadolinium-binding capabilities, fitting along with the rest of the acquired relaxivity data (Figure 3) in a statistically significant mannerConclusion
Biosynthesis of contrast agents such as the GLamouR have the potential to be utilized as a contrast agent for MRI and optical imaging as well as a probe for residual gadolinium reported through an increase of fluorescence. This system can eliminate several steps needed for quality control and synthesis, dramatically simplifying the process. Scaling up production while lowering costs via biosynthesis is being used in the pharmaceutical industry for therapeutics3 but has yet to be explored with molecular imaging probes.Acknowledgements
This project is supported by the following grants from the NIH/NIBIB: R01 EB031008; R01 EB030565; P41 EB024495References
1. Guo, B. J.; Yang, Z. L.; Zhang, L. J., Gadolinium Deposition in Brain: Current Scientific Evidence and Future Perspectives. Front Mol Neurosci 2018, 11, 335.
2. Salarian, M.; Turaga, R. C.; Xue, S.; Nezafati, M.; Hekmatyar, K.; Qiao, J.; Zhang, Y.; Tan, S.; Ibhagui, O. Y.; Hai, Y.; Li, J.; Mukkavilli, R.; Sharma, M.; Mittal, P.; Min, X.; Keilholz, S.; Yu, L.; Qin, G.; Farris, A. B.; Liu, Z. R.; Yang, J. J., Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat Commun 2019, 10 (1), 4777.
3. Kesik-Brodacka, M., Progress in biopharmaceutical development. Biotechnol Appl Biochem 2018, 65 (3), 306-322.
4. Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H. J., Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005, 40 (11), 715-24.
5. Szomolanyi, P.; Rohrer, M.; Frenzel, T.; Noebauer-Huhmann, I. M.; Jost, G.; Endrikat, J.; Trattnig, S.; Pietsch, H., Comparison of the Relaxivities of Macrocyclic Gadolinium-Based Contrast Agents in Human Plasma at 1.5, 3, and 7 T, and Blood at 3 T. Invest Radiol 2019, 54 (9), 559-564.
6. Noebauer-Huhmann, I. M.; Szomolanyi, P.; Juras, V.; Kraff, O.; Ladd, M. E.; Trattnig, S., Gadolinium-based magnetic resonance contrast agents at 7 Tesla: in vitro T1 relaxivities in human blood plasma. Invest Radiol 2010, 45 (9), 554-8.
7. Shen, Y.; Goerner, F. L.; Snyder, C.; Morelli, J. N.; Hao, D.; Hu, D.; Li, X.; Runge, V. M., T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol 2015, 50 (5), 330-8.
Figures