1987
Development of an image reconstruction algorithm to assess brain oxygenation in neonatal asphyxia using quantitative susceptibility mapping1NeuroPoly Lab, Institute of Biomedical Engineering, Polytechnique Montréal, Montreal, QC, Canada, 2Research Center, Ste-Justine Hospital University Centre, Montreal, QC, Canada, 3Department of Radiology, Radio-oncology, and Nuclear Medicine, Université de Montréal, Montreal, QC, Canada, 4Department of Computer and Software Engineering, Polytechnique Montréal, Montreal, QC, Canada
Synopsis
Neonatal asphyxia is a condition that causes lack of oxygen in the infant brain which lead to adverse neurodevelopmental outcomes. MRI provides a very clear image of the brain which lead to a detection of a variety of brain abnormalities. Quantitative susceptibility mapping (QSM) is a MRI tool to quantify brain oxygenation. In this study, we developed QSM reconstruction algorithms and tested them in data acquired in patients following neonatal asphyxia. Results include the comparison of five methods to generate QSM images in the brain specific segmented regions. Our preliminary data suggest that image intensity depends on the reconstruction method.
Introduction:
Neonatal hypoxic-ischemic encephalopathy (HIE) or birth asphyxia is a clinical syndrome of neurological dysfunction that occurs in 1 to 6 cases per 1000 live births [1]. The World Health Organization estimates that 38% of deaths of children under the age of 5 due to HIE at birth [2]. Currently, hypothermia therapy is the only treatment that showed a decrease in death [3]. Magnetic resonance imaging (MRI), and particularly quantitative susceptibility mapping (QSM), makes it possible to quantify brain oxygenation and therefore offers a relevant measure to assess brain integrity and response to therapy in newborns with HIE who underwent hypothermia [4]. However, current QSM image reconstruction methods were not developed for the developing brain which may affect the quantification of oxygenation levels. Additionally, the involuntary movements of the newborn can affect the quality of the images. QSM for neonatal imaging may require a more complex reconstruction process due to the ill-posed nature of the underlying problem and image artifacts. In this abstract, we present QSM images generated with five current techniques and compare oxygenation levels for the whole brain and specific structures.Methods:
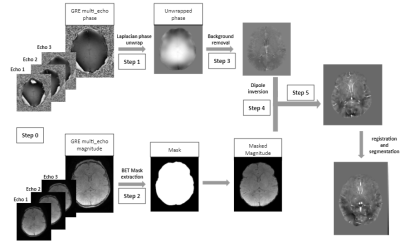
We acquired T1-weighted and multi-echo gradient-echo images from 5 patients (4 male) aged from 4 to 14 days in a 3T MRI scanner. Image acquisition consisted of a gradient echo sequence with a resolution of 0.41x0.41x1.6 mm3 and the following acquisition parameters: TR=0.0204/0.0223 s, TE=12, 15.38, 18.76 ms, flip angle=15°. Once the images have been acquired, the preprocessing steps include (Figure 1): (1) convert imaging data into phase and magnitude images, (2) unwrap phase that was encoded around [-π,+π], (3) create a mask to specify the region of interest (ROI) that includes only brain tissues, (4) remove background signal to isolate the external magnetic field of the ROI, and (5) apply dipole inversion to estimate the susceptibility of the brain and tissue oxygenation. Each step of the framework developed is then compared with existing methods (eg: MEDI, qMRLab) in terms of reconstruction quality. Reconstruction methods that were tested include: (1) Laplacian boundary value with rapid two step dipole inversion with sparsity priors (lbv_rts), (2) Regularization enabled sophisticated harmonic artifact reduction for phase data with morphology enabled dipole inversion (resharp_medi), (3) Method (2) with tikhonov regularization (resharp_tikh); (4): Method (2) with total variation deconvolution (resharp_tv); and (5) Projection onto dipole fields (pdf) using Rapid two step dipole inversion with sparsity priors (pdf_rts) for background removal and dipole inversion respectively [5]. Susceptibility maps are then individually co-registered in the T1-weighted structural space using a nonlinear registration method, using a morphological atlas of the neonatal brain, aged between 36 and 44 weeks. Once normalized, susceptibility maps are segmented using a hard segmentation algorithm for specific structures including deep gray nuclei, cerebro-spinal fluid (CSF), and cerebellum [6].Results:
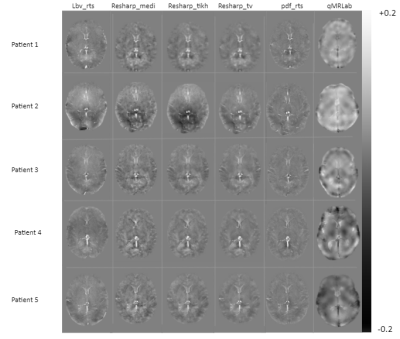
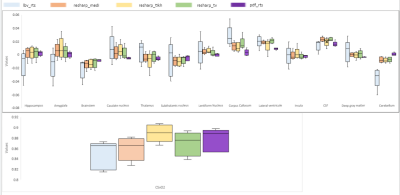
Figure 2 illustrates the reconstructed QSM image of five newborn patients (rows) using the preprocessing steps and the five reconstruction methods (columns) described above and a comparison with qMRLab. Figure 3 describes susceptibility values for specific segmented brain structures for each reconstruction method. It shows that for the lbv_rts, the variability between patients is higher than the other methods. For each segmented brain structure, the susceptibility values were similar for any of the methods used except for lbv_rts.Discussion / Conclusion:
Quantitative susceptibility mapping of the neonatal brain is challenging due to the motion of the newborn, the smaller brain structures and the shorter acquisition time [7]. This study compared specific image reconstruction algorithms. The results demonstrate robust QSM values in the neonatal brain, as shown by apparent small structures such as blood vessels.In addition, the susceptibility values and the oxygenation were in agreement with the literature [8]. Future work will include the development of new algorithms that are suited for neonatal brain images in order to select the best method in terms of reliability, reproducibility, speed and efficiency.Acknowledgements
This study was supported by Polytechnique Montréal, by the Canada First Research Excellence Fund, and by the TransMedTech Institute.References
1. Ferriero, Donna M. "Neonatal brain injury." New England Journal of Medicine 351.19 (2004): 1985-1995.
2. Aslam, Hafiz Muhammad, et al. "Risk factors of birth asphyxia." Italian journal of pediatrics 40.1 (2014): 1-9.
3. Shankaran, Seetha, and Abbot R. Laptook. "Hypothermia as a treatment for birth asphyxia." Clinical obstetrics and gynecology 50.3 (2007): 624-635.
4. Wang, Yi, et al. "Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care." Journal of Magnetic Resonance Imaging 46.4 (2017): 951-971.
5. Weber, A. M., et al. "Quantitative Susceptibility Mapping of Venous Vessels in Neonates with Perinatal Asphyxia." American Journal of Neuroradiology (2021).
6. Andreas Schuh, Antonios Makropoulos, Emma C. Robinson, Lucilio Cordero-Grande, Emer Hughes, Jana Hutter, Anthony N. Price, Maria Murgasova, Rui Pedro A. G. Teixeira, Nora Tusor, Johannes Steinweg, Suresh Victor, Mary A. Rutherford, Joseph V. Hajnal, A. David Edwards, and Daniel Rueckert, Unbiased construction of a temporally consistent morphological atlas of neonatal brain development, bioRxiv, 2018. doi: 10.1101/251512.
7. Dubois, Jessica, et al. "MRI of the neonatal brain: a review of methodological challenges and neuroscientific advances." Journal of Magnetic Resonance Imaging 53.5 (2021): 1318-1343.
8. Marques, José P., et al. "QSM reconstruction challenge 2.0: A realistic in silico head phantom for MRI data simulation and evaluation of susceptibility mapping procedures." Magnetic resonance in medicine 86.1 (2021): 526-542.
Figures