1971
Residual U-net for denoising 3D low field MRI1Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
Low magnetic field (LF) MRI is currently gaining momentum as a complementary, more flexible and cost-effective approach to MRI diagnosis. However, the impaired signal-to-noise ratio challenges its relevance for clinical applications. Recently, denoising of low SNR images using deep learning techniques has shown promising results for MRI applications. In this study, we assess the denoising performance of residual U-net architecture on different SNR levels of LF MRI data (0.1 T). The model performance has been evaluated on both simulated and acquired LF MRI datasets.
Introduction
Low-field (LF) MRI (<0.2 T) is gaining interest to complement current high-field systems with accessible scanners venturing outside of the radiology suite1. A common limitation of LF MRI lies however in the lower available spin magnetization generally yielding low Signal-to-Noise (SNR) images. During the image acquisition process, multiple averages (NA) can be used to boost the SNR by roughly $$$\sqrt{NA}$$$, although at the cost of prolonged acquisition times, compromising patient comfort and hindering the relevance of LF MRI for clinical routines. An alternative to signal averaging is denoising, and deep learning (DL)-based methods have recently shown promising results to that end2,3,4. In particular, residual5 U-net6, currently one of the state-of-the-art DL architectures, was successfully used in multiple denoising tasks7,8,9. However, its capability to denoise low SNR images such as those encountered at LF has never been explored. In this work, we evaluate the denoising performance of residual U-net in both simulated and acquired LF data, envisioning DL approaches as a compelling means to increase the clinical value of LF MRI.Materials and Methods
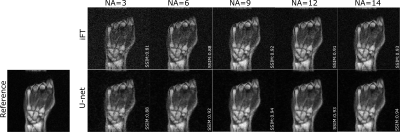
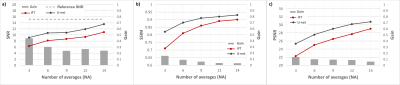
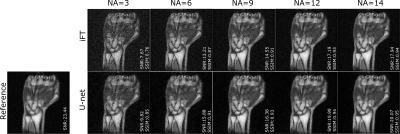
A total of 10 sets of 3D spoiled gradient echo (GRE) MR images of human wrists were acquired at 0.1T (4.25MHz) in a small footprint, non-shielded biplanar MRI system (EAR54L, Drusch & Cie, France)10. The following acquisition parameters were used: matrix=128×115×9, voxel size=[1.2×1.2×6.3]mm3, TE/TR=7.2/31ms, and NA=28 (acquisition time=14min56s). The training set consisted of high SNR images (i.e. acquired with the employed NA=28) paired with corresponding datasets, the SNR of which was artificially degraded. SNR-degradation was simulated by 1) rescaling k-space signal intensity, and 2) adding synthetic Gaussian noise to it in order to reach an SNR equivalent to NA=6. To limit overfitting issues, data augmentation was applied using a 3D version of the data generator function from the Keras library11. Residual U-net was trained using the RMSProp optimizer with an adaptive learning rate and the mean squared error as a loss function. To assess its performance, the model was first evaluated on 5 test sets of 3D wrist MR data, each one degraded to simulate five reduced NA=[3, 6, 9, 12, 14]. The images denoised with the residual U-net were eventually compared to the sole inverse Fourier transform (iFT) of the simulated noisy k-spaces. Ultimately, the model was validated on an acquired 3D MR dataset with NA=28, with each average individually stored in a fourth dimension; forming thereby a 4D MR dataset. This allowed retrospective manipulation of k-spaces to generate images with varying SNRs, from varying averaging rates. All images in the evaluation set were acquired with the same imaging parameters as described above. SNR, Structural Similarity Index (SSIM), and Peak SNR (PSNR) were chosen as evaluation metrics. In order to avoid an overestimation of the SNR and an underestimation of the SSIM and PSNR, the background noise was excluded from the computation of the quality metrics through masking. Finally, the relative increase in a given metric (gain) was reported.Results
Figure 1 shows an example of the model denoising performance in images of the human hand and wrist for different simulated NAs. Compared to the noisy images, residual U-net enhances image quality while preserving the texture, the contrast, and the details in the reconstructed images. Figure 2 presents the quantitative metrics obtained on the testing set with different simulated NAs. In all cases, U-net achieves improved SNR, SSIM and PSNR compared to the sole iFT of noisy k-spaces, particularly noticeable at lower NAs. Figure 3 demonstrates the denoising performance of the model on low field MR data (evaluation set) acquired using different NAs. A similar behavior to what observed in the simulation study can be seen.Discussion
Our results show that residual U-net can successfully denoise low SNR images with a significant gain in quantitative metrics, while preserving details (i.e., high spatial frequencies). Additionally, the model shows good generalization potential over a range of SNRs. We hypothesize that the latter may be a consequence of our training set, comprising images with heterogenous SNR levels. The equivalently good U-net performance demonstrated on acquired MR data is highly promising.Conclusion
In this work, we showed promising results for the denoising of 3D LF MRI data with residual U-net at various SNR levels, therefore opening alternatives to reduce NA and thus the acquisition time for LF MRI. Future work will be dedicated to further assess its performance in larger datasets for in vivo LF MRI and to compare residual U-net network to other existing DL denoising networks.Acknowledgements
This work is supported by the Swiss National ScienceFoundation Grant No. PP00P2_198905 and the university of Basel, faculty of medicine.
References
1Sarracanie et al. Low-Field MRI: How Low Can WeGo? A Fresh View on an Old Debate, Front Phys, 8:172 (2020)
2Jiang et al. Denoising of 3D magnetic resonance images with multi‑channel residual learning of convolutional neural network, Japanese Journal of Radiology, 36:566–574 (2018)
3Koonjoo et al. Boosting the signal‑to‑noise of low‑field MRI with deep learning image reconstruction, Scientific Reports, 11:8248 (2018)
4Ran et al. Denoising of 3D Magnetic Resonance Images Using a Residual Encoder-Decoder Wasserstein Generative Adversarial Network, Medical Image Analysis, 55:165-180 (2019)
5Lee et al. Deep Residual Learning For Compressed Sensing MRI, IEEE 14th International Symposium on Biomedical Imaging, (2017)
6Ronneberger et al. U-Net: Convolutional Networks for Biomedical Image Segmentation, arXiv:1505.04597, MICCAI,(2015)
7Heinrich et al. Residual U-Net Convolutional Neural Network Architecture for Low-Dose CT Denoising, Current directions in Biomedical engineering, 4(1):297-300 (2018)
8Ramos et al. A Residual Dense U-Net Neural Network for Image Denoising, IEEE Access, 9:31742-31754 (2021)
9Abscal et al. A residual U-Net network with image prior for 3D image denoising, 28th European Signal Processing Conference, 1264-1268 (2021)
10Constantinesco et al. Low-field dedicated and desktop magnetic resonance imaging systems for agricultural and food applications, Magn. Reson. Chem. 35: 69-75 (1997)
11https://keras.io/
Figures