1961
Automatic 3D to 2D reformatting in 4D flow MRI using continuous reinforced learning
Javier Bisbal1,2,3, Julio Sotelo1,3,4, Cristobal Arrieta1,3, Pablo Irarrázabal1,2,3, Marcelo Andia1,3,5, Cristian Tejos1,2,3, and Sergio Uribe1,3,5
1Biomedical Imaging Center UC, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2Electrical Engineering Department, School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 3ANID – Millennium Science Initiative Program – Millennium Nucleus for Cardiovascular Magnetic Resonance, Santiago, Chile, 4School of Biomedical Engineering, Universidad de Valparaíso, Valparaíso, Chile, 5Department of Radiology, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
1Biomedical Imaging Center UC, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2Electrical Engineering Department, School of Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 3ANID – Millennium Science Initiative Program – Millennium Nucleus for Cardiovascular Magnetic Resonance, Santiago, Chile, 4School of Biomedical Engineering, Universidad de Valparaíso, Valparaíso, Chile, 5Department of Radiology, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
One major limitation on 4D flow MRI is the time-consuming and user-dependent post-processing. We developed an automated reinforced deep learning framework for plane planning in 4D flow data. This method sequentially updates plane parameters towards a target plane based on a continuous policy. A total of 83 4D flow MRI scans were considered, 41 for training, 14 for validation and 28 for test. Our method achieves good results in terms of angulation and distance error (9.21 ± 3.85 degrees and 3.72 ± 2.19 mm).
Introduction
4D flow MRI allows the calculation of advanced hemodynamic parameters that provide valuable information to characterize cardiovascular diseases1. A considerable limitation is the user-dependent post-processing2. Specifically, manual positioning of planes along the major vessels involves a long time, particularly when analyzing several planes. Previous fully automated approaches include atlas registration3. However, atlas registration requires substantial computation time and depends on the similarity between the image and the atlas. Deep learning methods have been recently proposed to solve this issue4, and despite of the promising results, they have not fully exploited in terms of distance and angulation accuracy of the plane's planning. In this work, we adapted a continuous reinforced learning algorithm to get an action policy from a convolutional neural network (CNN) that sequentially moves and rotates an initial plane towards the target plane.Methods
We processed 4D flow data from GE, Siemens and Philips MRI scans of 67 healthy volunteers and 16 patients with congenital heart defects (8 with bicuspid aortic valve (BAV) and 8 with aortic coarctation) (47 men, 34 ± 12.4 years of age). All datasets were split in 50% training 20% validation and 30% testing, and preprocessed using an in-house MATLAB toolbox (MathWorks, Natick, MA, USA). The images used for the plane positioning corresponded to 43 PC-MRA5 and 40 complex difference6 extracted from 4D flow. The image pre-processing steps involved isotropic linear interpolation of 2x2x2 mm, segmentation of major vessels, contrast limited adaptive histogram equalization7 to reduce signal loss in angiographic data, and rigid registration to align all data in the same orientation. Then, we manually placed perpendicular planes through 4 vessels (pulmonary artery (PA), right pulmonary artery (RPA), left pulmonary artery (LPA) and ascending aorta (AAsc)) on the 3D visualization software Paraview (Kitware, Clifton Park, NY12065, USA). We modeled the search for the plane with a reinforced learning framework (Figure 1), where an initial state defined by a grid surrounding a specific plane in the 4D flow data is rotated and translated to reach a target location8. Each state is resampled from the volume and became the input of a 3D CNN that decided which actions to take on the plane's parameters. The goal is to maximize the reward function$$r=\left(D\left(P_{i-1}, P_{t}\right)-D\left(P_{i}, P_{t}\right)\right)+\lambda\left(M I\left(P_{i}, P_{t}\right)-M I\left(P_{i-1}, P_{t}\right)\right)$$
Where $$$P_i$$$ represents the plane in step $$$i$$$ and $$$P_t$$$ the target plane. $$$D$$$ is the Euclidean distance between the parameters of the two planes, and $$$MI$$$ the mutual information metric. The scalar $$$\lambda$$$ adjust the weight of each function and we fixed its value to 3. We trained the CNN following the Asynchronous Advantage Actor Critic9 (A3C) reinforced learning method. This algorithm builds a continuous action policy that allows a precise transition between states. We also randomly rotate and translate each train volume in each epoch to promote invariance to small location variations. In testing, we use a sequence of 100 steps and defined the last step as the estimated plane location where the CNN converge.
To measure the quality of the plane localization, we calculated the Euclidean distance between a point in the ground truth plane centered in the vessel and the estimated plane, and angulation error between the normal vectors. We also measure the structural similarity index (SSIM) and mutual information of both planes.
Results
The average plane mean and deviation were 9.21 ± 3.85 degrees of angle error and 3.72 ± 2.19 mm of distance error, which shows great positioning of the planes through the vessels (Figure 2). Also, the average mean and deviation of the SSIM were 0.70 ± 0.09 and 0.78 ± 0.15 for mutual information, which shows a high structure correlation between the manually defined and estimated planes. The results for the four plane locations are shown in Table 1.In terms of processing time, the longest step is the rigid registration that takes 20 seconds on average. The CNN plane search takes less than 5 seconds to find each plane, once trained. If the scans are acquired in the same orientation, registration is no longer needed, and with parallelization, processing time would take less than 5 seconds for all planes.
Conclusion
We developed a fast and automatic deep learning framework for plane planning on 4D flow data that is suitable for data acquired from different MRI scanners and between healthy volunteers and patients.Our results demonstrated a promising performance in terms of angulation and distance errors compared to other deep learning methods for 4D flow plane planning. According to the last reported method4 results, we improved the average accuracy of PA and AAsc plane planning by 11.0 and 4.4 mm for distance error, and 1.0 and 4.4 degrees for angle error, respectively. However, this comparison involves different test samples (40 for Corrado et al.4 and 28 for our method). Therefore, our next step will be to apply our method on additional planes and, ideally, on bigger public datasets for a fair benchmarking.
We can improve our method with other plane planning methods. For example, Corrado et al.4 finds 3D patches that isolates each vessel. If we use these patches instead of the full volume, the plane planning could be faster and more precise.
Acknowledgements
This work has been funded by ANID-PIA-ACT192064 and ANID – Millennium Science Initiative Program – NCN17_129. FONDECYT # 1181057, 1191710, 1180525. Sotelo J. thanks to FONDECYT de iniciación en investigación 11200481. Arrieta C. was partially funded by CONICYT FONDECYT Postdoctorado 2019 #3190763 and CONICYT PCI REDES 180090.References
- Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. Journal of Magnetic Resonance Imaging. 2012;36(5):1015-1036. doi:10.1002/jmri.23632
- Dyverfeldt, P., Bissell, M., Barker, A. J., Bolger, A. F., Carlhäll, C. J., Ebbers, T., ... & Markl, M. (2015). 4D flow cardiovascular magnetic resonance consensus statement. Journal of Cardiovascular Magnetic Resonance, 17(1), 1-19.
- Bustamante, M., Petersson, S., Eriksson, J., Alehagen, U., Dyverfeldt, P., Carlhäll, C. J., & Ebbers, T. (2015). Atlas-based analysis of 4D flow CMR: automated vessel segmentation and flow quantification. Journal of Cardiovascular Magnetic Resonance, 17(1), 1-12.
- Corrado, P. A., Seiter, D. P., & Wieben, O. (2021). Automatic measurement plane placement for 4D Flow MRI of the great vessels using deep learning. International Journal of Computer Assisted Radiology and Surgery, 1-12.
- Markl, M., Kilner, P. J., & Ebbers, T. (2011). Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance, 13(1), 1-22.
- Bernstein, M. A., & Ikezaki, Y. (1991). Comparison of phase‐difference and complex‐difference processing in phase‐contrast MR angiography. Journal of Magnetic Resonance Imaging, 1(6), 725-729.
- Reza, A. M. (2004). Realization of the contrast limited adaptive histogram equalization (CLAHE) for real-time image enhancement. Journal of VLSI signal processing systems for signal, image and video technology, 38(1), 35-44.
- Alansary, A., Le Folgoc, L., Vaillant, G., Oktay, O., Li, Y., Bai, W., ... & Rueckert, D. (2018, September). Automatic view planning with multi-scale deep reinforcement learning agents. In International Conference on Medical Image Computing and Computer-Assisted Intervention (pp. 277-285). Springer, Cham.
- Mnih, V., Badia, A. P., Mirza, M., Graves, A., Lillicrap, T., Harley, T., ... & Kavukcuoglu, K. (2016, June). Asynchronous methods for deep reinforcement learning. In International conference on machine learning (pp. 1928-1937). PMLR.
Figures
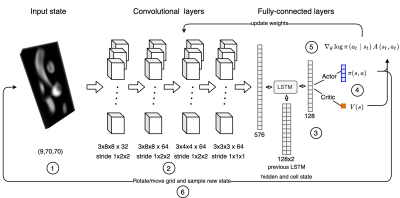
Figure 1: Reinforced learning framework for 4D flow plane search. An initial stack of slices (1) pass through 4 convolutional layers (2) and 1 fully connected layer. The output of this layer enters a Long short-term memory cell (LSTM) with the previous LSTM hidden and cell state (3). The LSTM calculates the policy (π(a,s)) and value function (V(s)) (4). CNN parameters are updated with the equation (5) where A(s,a) is the advantage function of the A3C algorithm. Finally, the policy rotates and moves the grid to reach a new state (6).
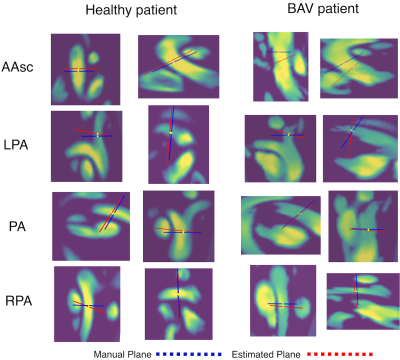
Figure 2: Plane localization results shown as lines on both orthonormal images of the ground-truth plane. Estimated planes are shown in red and ground truth plane in blue. The yellow points were used to measure the distance error between planes.
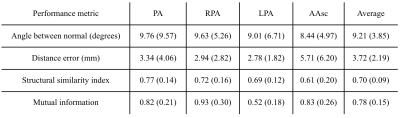
Table 1: Performance metrics in test patients. Average value in each plane and standard deviation in parenthesis. The last column averages the metrics on all planes and then over all patients.
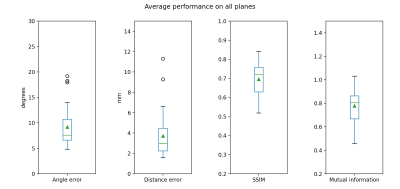
Figure 3: Performance metrics in test patients averaged on all planes.
DOI: https://doi.org/10.58530/2022/1961