1958
Gaussian Process Modelling and Compensation of Motion in DCE-MRI1Radiology, Boston Children's Hospital, Boston, MA, United States
Synopsis
Radial DCE-MRI is robust to motion. However, bulk motion or heavy breathing causes 1) irrecoverably deteriorated k-space lines acquired during motion events reducing image quality, 2) misaligned volumes in a dynamic sequence. In this work we propose to solve the first problem by fitting a Gaussian process to the k-space center of each spoke over time and using it to determine outlier spokes corrupted by motion. We solve the second problem by clustering the dynamic data to respective motionless phases before and after each motion event and registering volumes between phases for computationally efficient correction of motion with fewer registrations.
Introduction
Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) in abdomen is subject to breathing and bulk motion that causes (1) irrecoverably deteriorated k-space lines acquired during motion events reducing image quality, (2) misaligned volumes in a dynamic sequence. Motion changes measured signal from multiple coils differently and this allows us to detect the motion from multi-coil k-space center. Once motion is detected, motion artifacts are compensated by removing/adjusting damaged spokes to solve (1) and by registering misaligned clusters/phases of volumes to solve (2). Previously, cross-correlation (Crr) between measurements were used to represent their dependence and to detect outliers when Crr drops below a threshold [1, 2]. These approaches are subject to high false alarm rate as the Crr is computed within a window around each respective time-point. Additionally, such methods fail to separately detect problems (1) and (2).Here we propose a motion detection method that is capable of detecting both outliers and possible misalignments. To solve (1), we fit a Gaussian process model (GPM) to the k-space center of each spoke over time and use its log-likelihood to determine outlier spokes corrupted by motion. To solve (2), we cluster the dynamic data into motionless phases before and after each motion event and we correct misalignments by performing registration only between phases. Our results showed improved motion correction performance compared to previous methods.Method
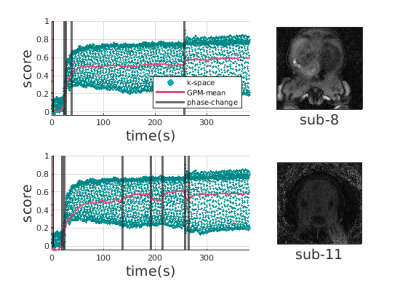
We acquired coronal DCE-MR images with a dynamic stack of stars sequence with golden angle radial sampling scheme from 14 patients (10 female, 4 male, age=7.7$$$\pm$$$7.5 years) with temporal resolution (~3.3s/volume) [2] following an approved IRB protocol and after obtaining consent.We construct temporal feature vectors by concatenating center of k-space samples from all coils (after coil compression using PCA) for each time sample. We then fit a noisy GP with a radial basis function (RBF) kernel. Thresholding negative log likelihood scores is used to detect outliers. We removed these outlier spokes during our motion-compensated image reconstruction.To divide temporal data into motion-free phases/clusters, we use principle component analysis and take the most significant component of the projected $$$N$$$ dimensional GP mean vector reducing it to a univariate vector. The intensity of the reduced mean is an indicator of activity through the scan and hence, it is possible to cluster time points with a simple k-means approach. With motion resulting in misalignments, we expect a shift on the reduced mean. Figure-1 illustrates the mean of the GPM that is fitted to the center of k-space lines over time. Blue dots represent center of k-space lines, red curves represent GPM mean and black vertical lines represent separators between clusters. Top row (subject-8) demonstrates the performance of the model under bulk motion, bottom row (subject-11) illustrates the performance of the model both under bulk motion and some irrecoverable samples acquired during motion. Observe that for both examples, clusters are either separated by contrast differences or they correspond to different motion phases. We compare our method to Crr based motion detection algorithms [1, 2], where Crr is computed within a window.Results
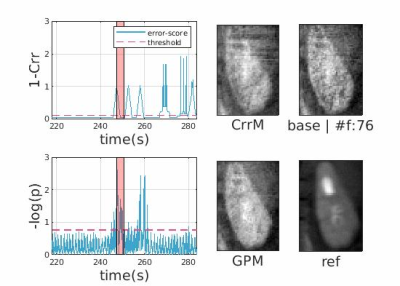
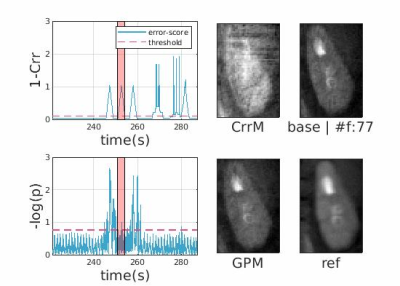
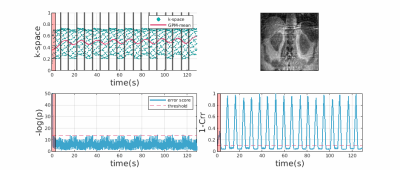
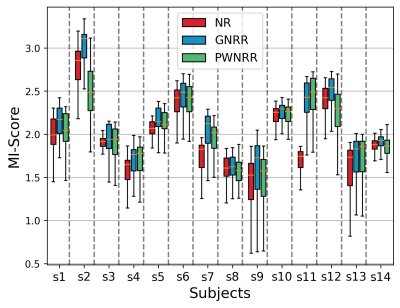
Figures-[2,3], each contains the following: Two score plots (top: negative correlation, bottom: negative log likelihood) and their respective thresholds used to discard outlier spokes. Figure shows four cropped kidney regions from different reconstructions (CrrM: cross-correlation-model, GPM: Gaussian-process-model, base: no-outlier-rejection, ref: densely-sampled-reconstruction static reconstruction). Figure-2 shows that GPM effectively removes outlier spokes corrupted by motion without completely losing the kidney signal where CrrM perturbs adrenal region. Observe from Figure-3, GPM only removes 1 corrupted spoke and slightly recovers the degraded signal, whereas CrrM distributes rejection to neighboring samples (due to windowing effect) and hence removes possibly good spokes as well. We exemplify performance comparison of GPM and CrrM methods on a non-contrast volunteer data with periodic heavy breathing motion in Figure-4. The kspace center signal was used to divide the respiratory signal into phases previously in literature [3]. Observe that GPM mean follows this periodic behavior and can divide the data into respiratory phases more effectively compared to CrrM.Figure-5 summarises the mutual information (MI) scores [4] for each dynamic image in the series that is the MI values between each temporal image $$$x_{t}$$$ and a densely-sampled reference image $$$x_{\text{ref}}$$$. We show box-plots of MI scores for each subject for no-registration (NR) , group-nonrigid-registration (GNRR), pair-wise-nonrigid-registration (PWNRR) after outlier spoke rejection. We observe that registration on average improves MI. However, for subjects where there are dynamic time images with majority of the spokes are rejected (e.g. sub-2), computing respective pairwise registration map results amplified noise and furthermore reducing the MI values. We note group-nonrigid-registration performs at par to pair-wise-nonrigid-registration while reducing number of non rigid registrations from ~160 to 5 in a 6(mins) acquisition.Conclusion and Discussion
Proposed GPM can effectively detect outlier spokes degraded by motion and dis robust to false alarm rates by being more selective in outlier rejection compared to CrrM. Moreover, GPM allows clustering temporal data into motionless phases that are further aligned by non-rigid registration to increase the motion-compensated image reconstruction quality as indicated by the increased MI scores with the reference image from a densely sampled reconstruction.Acknowledgements
This work was supported partially by the Society of Pediatric Radiology Multi-center Research Grant 2019, Crohn’s and Colitis Foundation of America’s (CCFA) Career Development Award and by the NIDDK and NIBIB of the National Institutes of Health under award numbers R01DK125561, R21DK123569, R21EB029627, and by the grant number 2019056 from the United States-Israel Binational Science Foundation (BSF), and a pilot grant from National Multiple Sclerosis Society under Award Number PP-1905-34002, and Society of Pediatric Radiology, Multicenter Research Award.References
[1] Caparelli, Elisabeth C., et al. "k-Space based summary motion detection for functional magnetic resonance imaging." Neuroimage 20.2 (2003): 1411-1418.
[2] Coll‐Font, Jaume, et al. "Bulk motion‐compensated DCE‐MRI for functional imaging of kidneys in newborns." Journal of Magnetic Resonance Imaging 52.1 (2020): 207-216.
[3] Feng, Li, et al. "XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing." Magnetic resonance in medicine 75.2 (2016): 775-788.
[4] Maes, Frederik, Dirk Vandermeulen, and Paul Suetens. "Medical image registration using mutual information." Proceedings of the IEEE 91.10 (2003): 1699-1722.
Figures