1954
Accelerated 3D EPI navigator for prospective motion correction1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Boston, MA, United States
Synopsis
We show that for navigator-based prospective motion correction MRI, acceleration of 3D EPI acquisition increases sequence flexibility and improves the navigator image quality without sacrificing the quality of motion correction.
Introduction
Navigator-based prospective motion correction (PMC) has become indispensable in MRI to manage motion artifact in both clinic and research1-3. This approach is relatively easy to set up as it does not reply on additional hardware for motion tracking. However, the real-time navigator acquisitions require “dead time” in the MRI pulse sequence and this imposes limits on the sequence timing. For 3D EPI-based volumetric navigators (vNavs), in addition to the extended time needed for the full 3D volume acquisition, the prolonged echo train for each partition also results in signal loss and image distortion. Accelerated EPI acquisitions have shown improved imaging speed and image quality4. In this study, we explore the impact of accelerating the navigator on sequence timing, navigator image quality, and real-time motion correction for structural imaging of the brain.Methods
The prototype MR pulse sequence used in this study consists of MPRAGE5 as the “parent” sequence and 3D-EPI as the navigator in each TR between the inversion RF pulse and the turbo-FLASH readout3. Details of the MPRAGE: 1 mm isotropic resolution, sagittal orientation, 240×210×160 matrix (with 10% oversampling along partition), phase-encoding acceleration factor of 2, phase partial Fourier 6/8; TR=2.5 s, 4-echo readout6 with TE=2.08/3.84/5.60/7.36 ms and bandwidth of 720 Hz/pixel. The navigator details: 256 mm cubic FOV and 32×32×32 matrix; a single-shot EPI readout for each partition; partition partial Fourier 6/8; binomial water excitation RF pulses of 2° or sinc RF of 1°; readout with ramp sampling and bandwidth of 5040 Hz/pixel.An in-plane acceleration factor of 3 is used to reduce the single-shot EPI echo train from 32 echoes to 11 echoes for each navigator partition, which reduced TE from 6.68 ms to 3.60 ms and the total readout duration from 8.96 ms to 3.08 ms. An additional acceleration factor of 2 along partition was also used in selected scans to reduce the total navigator acquisition time. GRAPPA7 is used to reconstruct the accelerated navigators in real-time based on the external GRE reference scan at the beginning of the sequence. An anthropomorphic head phantom8 and two volunteers were scanned on a 3 T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) whole-body scanner with a 32-ch receive head coil. A written consent was obtained from each volunteer. Volunteers were instructed to move during the scan.
Results
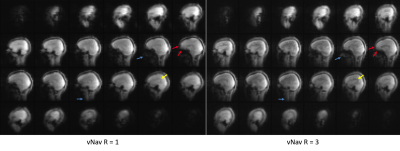
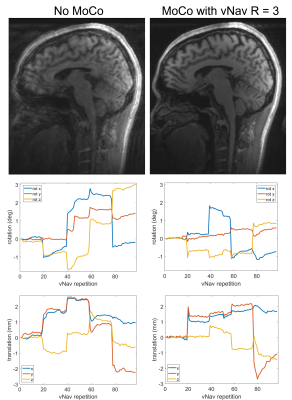
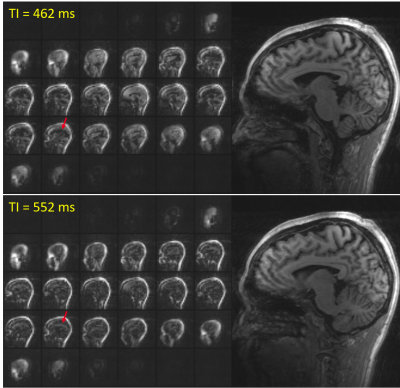
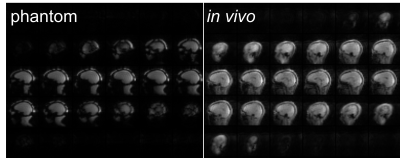
With 3× in-plane acceleration, the total navigator acquisition is reduced from 272 ms to 125 ms (sinc pulse; from 355 ms to 208 ms for water excitation), and to just 65 ms when combined with 2× partition acceleration. The shortened navigator allows shorter TI (≤1100 ms) for MPRAGE to optimize the gray-white matter contrast6 compared to the minimum allowed TI (1260 ms) with no acceleration.Figure 1 compares the image quality between fully sampled and 3× accelerated navigators. For the fully sampled navigators, signal dropout is visible for areas of high susceptibility, whereas the signals in these areas are visibly improved for the accelerated navigators (red arrows). In addition, due to the effective TI change (from 247 ms to 156 ms), the accelerated navigator exhibits enhanced gray-white matter contrast (yellow arrow). The utility of the accelerated navigator for PMC is shown in Fig. 2, where two scans with moderate motions were acquired. Clearly, PMC using the 3× accelerated navigator significantly improves image quality compared to the one without motion correction. As shown in Fig. 3, at 3× acceleration, different gray-white matter contrasts can be obtained over the extended range of effective navigator TIs allowed by partition partial Fourier of MPRAGE, even though the overall brain signals are suppressed (similar to FatNav9). These navigators, despite their different appearance, are all shown to be effective in reducing motion artifact with PMC. Figure 4 shows phantom and in vivo navigator images acquired with the additional 2× partition acceleration. Note at this level of acceleration (6×) there is nearly no discernible parallel imaging artifact or g-factor10 penalty.
Discussion
Acceleration of the navigator acquisition in PMC provides several benefits. Most importantly, the reduced temporal footprint requires less dead time in the sequence, rendering more flexibility in the timing of a sequence and potentially allowing vNav to be used in more sequences. Specific for MPRAGE, this translates into shorter TI for contrast optimization. Second, geometric distortion of the navigator is substantially reduced in proportion to the acceleration factor. Third, signal dropouts are reduced by the shorter navigator TE. Finally, since the navigator itself is acquired on the T1 recovery curve, a steady state can never be assumed. Therefore, a shorter acquisition window leads to less image and contrast blurring. For MPRAGE, our data showed that the navigator should be acquired closer to the inversion pulse in order to maximize SNR.Conclusions
In this study we demonstrated that accelerated navigators allow for more flexibility of sequence timing and improve navigator image quality without compromising the motion correction functionality.Acknowledgements
No acknowledgement found.References
1. White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, Dale A, PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking, Magn Reson Med, 2010;63:91-105
2. Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, van der Kouwe AJW, Real-time Motion and B0 Corrected Single Voxel Spectroscopy Using Volumetric Navigators, Magn Reson Med, 2011;66:314-323
3. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW, Volumetric Navigators for Prospective Motion Correction and Selective Reacquisition in Neuroanatomical MRI, Magn Reson Med, 2012;68:389-399
4. de Zwart JA, van Gelderen P, Golay X, Ikonomidou VN, Duyn JH. Accelerated parallel imaging for functional imaging of the human. NMR Biomed 2006;19:342–351
5. Mugler JP III, Brookeman JR, Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE), Magn Reson Med, 1990;15:152-157
6. van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage 2008;40:559–569.
7. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A, Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn Reson Med, 2002;47:1202-1210
8. Graedel NN, Polimeni JR, Guerin B, Gagoski B, Wald LL, An anatomically realistic temperature phantom for radiofrequency heating measurements. Magn Reson Med, 2015;73:442-450
9. Skare S, Hartwig A, Martensson M, Avventi E, Engstrom M, Properties of a 2D Fat Navigator for Prospective Image Domain Correction of Nodding Motion in Brain MRI. Magn Reson Med, 2015;73:1110-1119
10. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P, SENSE: Sensitivity Encoding for Fast MRI. Magn Reson Med, 1999;42:952-962
Figures