1951
Evaluation of markerless prospective motion correction for neuroanatomical MRI
1Institute of Cognitive Neuroscience Marc Jeannerod, CNRS / UMR 5229, BRON, France, Metropolitan, 2Université Claude Bernard - Lyon 1, Villeurbanne, France, Metropolitan, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Cermep, CNRS / UAR 3453, Lyon, France, Metropolitan, 6Siemens Healthcare SAS, Saint-Denis, France, Metropolitan
Synopsis
Head-motion is a main cause of artifacts in MRI. The performance of a markerless optical system, which records the subject's face, estimates head-motion, and allows real-time repositioning of the FOV, is evaluated for neuroanatomical MRI. Sets of T1W/T2W-images were collected from 2 subjects instructed to perform different head-motion protocols during the acquisitions. The System performance is evaluated by comparing images with/without motion-correction. The optical system ensures good corrections of head-motions. However, the correction quality depends on the amplitude of the movement, its location in k-space and its nature. Residual effects of large amplitudes/amounts movements may persist on corrected images.
Introduction
Motion artifacts are a major concern in MRI1. Several strategies using a navigator system have been developed to correct head motion for anatomical MRI of the brain2,3. Optical head position tracking systems have recently been introduced to perform real-time motion correction during cerebral MRI4,5,6.The aim of this study is to evaluate the performance of markerless prospective motion correction (PMC)5 as a function of the location, amount, and amplitude of head movements occurring during anatomical MRI.
Materials and methods
MRI acquisitions were performed on a MAGNETOM Prisma 3T scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel receive coil. A commercial markerless motion tracking system5, which uses a camera to capture the subject’s face and assess the head translations and rotations 30 times per second, was used in this study. The anatomical sequences are interfaced to the motion correction system in order to real-time update the field-of-view (FOV) of the acquisition to the new head position after the scanning of each 6 lines of k-space. TRs whose head-motion scores7 exceeded a fixed threshold are re-acquired8,9.A cross-calibration phase is required at the beginning of each MRI session before motion estimation starts. This calibration allows the PMC optical system to match its coordinates to those of the MRI using a rapid slab-selective scan of the face.
T1-weighted (T1W) images were collected using a prototype MP-RAGE sequence with a FOV of 256×256×176 mm3, TR/TE=2500/3.33 ms, FA=8°, a TI=1070 ms, BW=240Hz/Px, GRAPPA acceleration-factor=2, turbo-factor of 176, resulting in a total acquisition time of 5:59-minute. The echo spacing was 8.1 ms, which implies an update of the FOV position every 48.6 ms.
T2-weighted (T2W) images were collected using a prototype T2-SPACE sequence with a FOV of 256×256×176 mm3, a TR/TE of 3200/565 ms, BW=241 Hz/Px, and GRAPPA=2, a turbo factor of 200, an echo spacing of 6.3 ms and T2Var refocusing mode. The FOV update was applied every 37.8 ms. The total acquisition time of T2W-images was 5:34-minute.
To reproduce the head movements during the 2 experiments carried here, we designed visual cues for head-motion protocols using the Presentation tool (Neurobehavioral Systems).
Experiment 1: Five different head-movement protocols (Figure 1. A and Prot.1-Prot.5) were executed by two subjects during the MRI acquisitions to test the ability of correction as a function of motion amplitude, the part of k-space being acquired and quantity of motion. Subjects were scanned 8 times with the MP-RAGE and T2-SPACE sequences. 5 images were acquired with PMC during the 5 motion-protocols, 2 images were collected without PMC and with motion-protocols 1 and 2, and a reference image was acquired without movement.
Experiment 2: To investigate the limitations of markerless PMC, T1W and T2W image series of a subject repetitively performing continuous and discontinuous head motions were acquired (Figure 1. B). It should be noted that this pattern of movement may never occur in the clinical routine.
Results
The rotation and translation curves measured by the markerless optical system during the different MRI acquisitions revealed the intra-subject reproducibility of the head movements guided by the "Presentation" software.Images acquired with protocol 1 (head-motions of ±6° performed at the beginning of the scan) and without prospective motion correction exhibited minor artifacts compared to protocol 2 (head-movements of ±6° executed at the acquisition half-time) for both MP-RAGE and T2-SPACE (Figure 2. A). The effects of motion protocols 1 and 2 appear to be completely compensated by the PMC.
PMC adequately corrected head rotations up to ±15° produced at the beginning (Prot. 3) and halfway through the acquisition (Prot. 4 - Figure 2. B).
Discontinuous and continuous head movements severely impacted the quality of T1W and T2W images acquired without PMC in Experiment 2 (Figure 3). For MP-RAGE, the PMC demonstrated good performance in the correction of discontinuous motion, while some artifacts persisted in the continuous motion corrected image. As for the quality of the T2-SPACE images correction, PMC has significantly reduced the effects of discontinuous movements, despite their extreme severity (Figure 3. C-D).
Discussion
The work presented in this report validated the results obtained by Frost et al.10 which demonstrate the ability of markerless PMC to compensate in real-time the effects of head-movements on brain anatomical MRI. This study shows that the ability of the markerless PMC system to correct head movements may slightly decrease when these movements are intense and coincide with the sampling of the k-space center. Repeated large-amplitude head motions throughout the acquisition represent a challenge for the markerless PMC system, especially when these movements are continuous, and can lead in the most extreme cases to T2-SPACE PMC failure. However, this pattern of movement is very rare and may not arise in the clinical routine.Conclusion
The markerless optical PMC system has demonstrated good performance for head motion correction on T1 or T2 weighted anatomical MRI. However, the quality of the correction depends on the magnitude of the motion, its location in k-space and the nature of the motion (continuous or discontinuous). Residual effects of large amplitudes/quantities motions were detected in the corrected images. The PMC was slightly less effective in correcting T2-SPACE images for large continuous motion.Acknowledgements
No acknowledgement found.References
1. J. Andre, B. Bresnahan et M. Mossa - Basha, «Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations» J Am Coll Radiol., vol. 12, pp. 689-695, 2015.
2. M. D. Tisdall, M. Reuter, A. Qureshi, R. L. Buckner, B. Fischl et A. J. v. d. Kouwe, «Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion» Neuroimage, vol. 127, pp. 11-22, 2016.
3. S. Skare, A. Hartwig, M. Mårtensson, E. Avventi et M. Engström, «Properties of a 2D fat navigator for prospective image domain correction of nodding motion in brain MRI» Magnetic Resonance in Medicine, vol. 73, n°13, pp. 1110-1119, 2015.
4. «KinetiCor,» [En ligne]. Available: https://kineticor.com/about. [Accès le 27 08 2021].
5. J. M. Slipsager, A. H. Ellegaard, S. L. Glimberg, S. L. Paulsen, D. M. Tisdall, P. Wighton, A. Van Der Kouwe, L. Marner, O. M. Henriksen, I. Law et O. V. Olesen, «Markerless motion tracking and correction for PET, MRI, and simultaneous PET/MRI», 2019.
6. Gretsch F, Mattern H, Gallichan D, Speck O. Fat navigators and Moire phase tracking comparison for motion estimation and retrospective correction. Magnetic Resonance in Medicine. 2020; 83:83–93. https ://doi.org/10.1002/mrm.27908.
7. J. Mark, «Measuring Transformation Error by RMS Deviation. » Technical Report. FMRIB Technical Report. Oxford: FMRIB., 1999.
8. N. White, C. Roddey, A. Shankaranarayanan, E. Han, D. Rettmann, J. Santos, J. Kuperman, and A. Dale. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med, 63(1):91–105, 2010.
9. M. D. Tisdall, A. T. Hess, M. Reuter, E. M. Meintjes, B. Fischl, and A. J. W. van der Kouwe. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med, 68(2):389–399, 2012.
10. R. Frost, P. Wighton, F. I. Karahanoğlu, R. L. Robertson, E. P. Grant, B. Fischl, D. M. Tisdall et A. Van der Kouwe, «Markerless high‐frequency prospective motion correction for neuroanatomical MRI» Magnetic Resonance in Medicine, vol. 82, pp. 126-144, 2019.
Figures
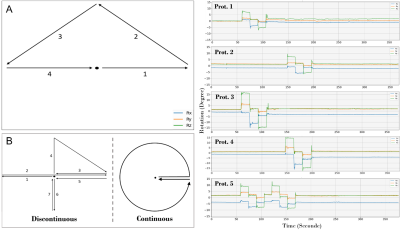
Figure 1. (A) - Schema of the 4 head-motions of 1 second each of experiment 1. (B) - Diagram of continuous and discontinuous head-motions of experiment 2. (Prot.) - Head rotations measured by the optical PMC system for the 5 motion-protocols over the entire acquisition time.
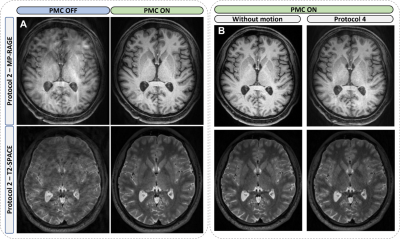
Figure 2. Examples of T1W (top) and T2W (bottom) images obtained in Experiment 1 with and without prospective motion correction (PMC). PMC allowed an effective correction of the 4 head-motions of ±6° (Prot.2) and ±15° (Prot.4) executed in the middle of the acquisition (during the sampling of k-space center).
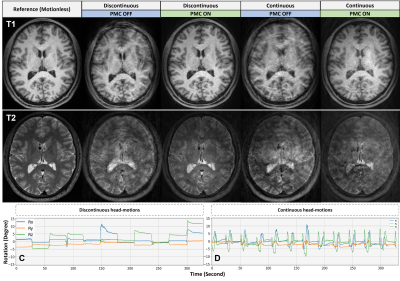
Figure 3. T1W and T2W images obtained in Experiment 2 with and without prospective motion correction (PMC). The effects of head-motions (±5° to ±13°) occurring during the entire MP-RAGE acquisition were corrected by PMC with residual artifacts and blurring for continuous movements. As for the T2-SPACE images, PMC significantly reduced the effects of discontinuous motions (C) but failed to compensate for the effects of continuous motions (D) that may never occur in the clinical routine.