1949
Evaluation of Scout Accelerated Motion Estimation and Reduction (SAMER) for measurement of brain volume and cortical thickness1Siemens Medical Solutions, Boston, MA, United States, 2Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Scout Accelerated Motion Estimation and Reduction (SAMER) is a novel technique that can mitigate motion artifacts in MR images. In this study, we compared the brain volume and cortical thickness measurements obtained from in vivo motion-free, motion corrupted, and motion corrected images acquired with SAMER to those from a motion-free standard MPRAGE reference scan. The quantitative analysis of motion-free and motion corrected SAMER images showed comparable results with motion-free standard MPRAGE.
Introduction
Volumetric brain MRI is a powerful tool for the evaluation of patients with neurodegenerative diseases, providing quantitative biomarkers that can be useful in both clinical and research settings [1,2]. For example, hippocampal volume inversely correlates with disease severity in patients with mild cognitive impairment and predicts the development of Alzheimer disease [3], while cortical thickness measurements in patients with Parkinson's disease correlate with the clinical stage of disease and associated dementia [4]. The MRI examination protocol for such studies typically consists of a 3D T1-weighted MPRAGE acquisition (T1w-MPRAGE) that provides high spatial resolution and tissue contrast [5], followed by automated image segmentation and post-processing using software such as FreeSurfer (http://surfer.nmr.mgh.harvard.edu). However, elderly patients are prone to motion artifacts, which may degrade the accuracy of volumetric segmentation [6-8].Recently, Scout Accelerated Motion Estimation and Reduction (SAMER) has been proposed and applied to mitigate motion artifacts in MR sequences, including T1w-MPRAGE [9]. SAMER combines a fast (3-5 second) scout acquisition with an optimized k-space reordering to provide data-driven estimates of the rigid body motion parameters for each k-space shot and provides a robust and computationally efficient framework for retrospectively reconstructing an artifact free image.
In this study, we hypothesized that head motion would degrade the accuracy of brain volume and cortical thickness measurements obtained using FreeSurfer, and that use of the SAMER approach for motion mitigation could improve the accuracy of these measurements in the presence of motion.
Methods
Three MPRAGE scans were acquired in vivo at R=4-fold acceleration in an informed and consented healthy volunteer: (1) motion-free standard reference, (2) motion-free SAMER acquisition, and (3) motion corrupted SAMER acquisition. All scans were acquired on a 3T system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil. The sequence parameters used were: FOV 256x256x192mm3; matrix 256x256x192; TR/TE/TI 2300/3.3/1000ms; flip angles 8°; bandwidth 200 Hz/Px. The acquisition times were 2:32 min for standard and 2:42 min for the SAMER acquisitions, respectively. The volunteer was instructed to perform deep breathing during the motion scan, which is known to introduce motion artifact. The motion estimation and image reconstruction were performed with an online reconstruction directly on the scanner and generated additional motion corrected DICOM images.The FreeSurfer tool [10] was used for automated human brain segmentation and parcellation of four standard DICOM image volumes. Regional brain volume and cortical thickness measurements from 11 different brain regions (frontal, parietal, temporal, and occipital lobes; cingulate gyrus; insula; hippocampus; basal ganglia; brain stem; cerebellum; and cerebral white matter) were extracted for quantitative analysis. The percentage error in the volume and cortical thickness measurements was calculated for the SAMER images with respect to the standard reference scan.
Results and Discussion
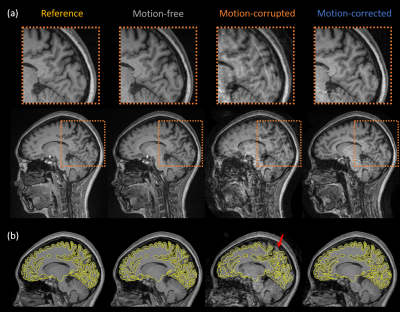
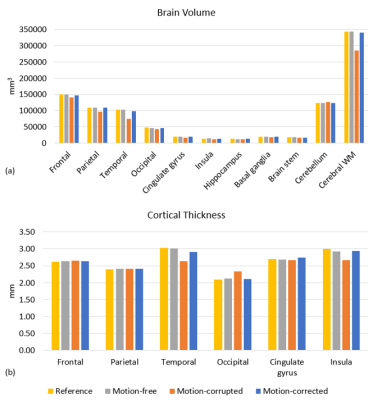
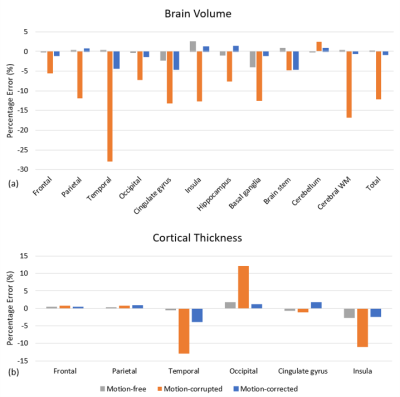
Figure 1 shows representative slices from each of the image volumes and indicates that the image quality of motion-free and motion-corrected SAMER was comparable to the standard reference scan. Significant motion artifacts present in the motion-corrupted image are substantially reduced after SAMER motion correction. Figure 1b shows the pial and gray-white surface outlines from the FreeSurfer segmentation superimposed on the corresponding images. As can be seen, the segmentation of the motion-free and motion-corrected SAMER images showed excellent agreement with the reference, while segmentation of the motion-corrupted scan produced a smaller contour which potentially excluded some brain tissues.Substantial reduction in the estimated brain volume was observed in the frontal, parietal, temporal lobes, and cerebral white matter of the motion-corrupted scan (Figure 2a). Differences in cortical thickness (Figure 2b) were found in the temporal and occipital lobe and insula regions. The highest difference in estimated brain volume (Figure 3a) was in the temporal lobe, where the percentage error for motion-corrupted images was 28.0%, and 0.3% and 4.3% for motion-free and motion corrected images. The lowest difference was in the cerebellum, where the percentage error for motion-corrupted images was 2.4%, and 0.0% and 0.8% for motion-free and motion corrected images. The motion-corrupted images showed the largest differences of brain volume in all segmented brain regions and larger differences of cortical thickness (>11.0%) in temporal lobe, occipital lobe and insula region. The percentage error of cortical thickness was slightly larger in motion-corrected images as compared to motion-corrupted images in the parietal lobe and cingulate gyrus, but the differences in both regions were less than 1.7%. The brain volume and cortical thickness estimates extracted from motion-corrupted images were biased by head motion, and the SAMER technique improved the accuracy of brain volume and cortical thickness measurements in the presence of motion. Our finding matched to the results reported in [11] that the apparent brain volume loss was associated with motion. Validation in larger studies including motion prone clinical populations is warranted.
Conclusion
This preliminary study indicates that brain volumes and cortical thickness estimated using the SAMER motion correction technique were comparable with those estimated using motion-free standard MPRAGE. SAMER may be helpful in volumetric studies involving motion prone patients including elderly patients with neurodegenerative diseases.Acknowledgements
This work was supported in part by NIH research grants: 1P41EB030006-01, 5U01EB025121-03 and Siemens Healthineers.References
1. Rusinek H, de Leon MJ, George AE, et al. Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology. 1991;178(1):109-114. doi:10.1148/radiology.178.1.1984287
2. Lehmann M, Douiri A, Kim LG, et al. Atrophy patterns in Alzheimer’s disease and semantic dementia: a comparison of FreeSurfer and manual volumetric measurements. Neuroimage. 2010;49(3):2264-2274. doi:10.1016/j.neuroimage.2009.10.056
3. Frisoni GB, Fox NC, Jack CR, Scheltens P, Thompson PM, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67-77. doi:10.1038/nrneurol.2009.215
4. Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84(8):875-882. doi:10.1136/jnnp-2012-304126
5. Mugler JP, Brookeman JR. Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging. 1(5):561-567. http://www.ncbi.nlm.nih.gov/pubmed/1790381. Accessed June 30, 2019.
6. Munn Z, Pearson A, Jordan Z, Murphy F, Pilkington D, Anderson A. Patient anxiety and satisfaction in a magnetic resonance imaging department: Initial results from an action research study. J Med Imaging Radiat Sci. 2015;46(1):23-29. doi:10.1016/j.jmir.2014.07.006
7. Havsteen I, Ohlhues A, Madsen KH, Nybing JD, Christensen H, Christensen A. Are movement artifacts in magnetic resonance imaging a real problem?-a narrative review. Front Neurol. 2017;8(MAY):1-8. doi:10.3389/fneur.2017.00232
8. Savalia NK, Agres PF, Chan MY, Feczko EJ, Kennedy KM, Wig GS. Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum Brain Mapp. 2017;38(1):472-492. doi:10.1002/hbm.23397
9. Polak D, Splitthoff DN, Clifford B, et al. Scout accelerated motion estimation and reduction (SAMER). Magn Reson Med. 2021. Epub ahead of print.
10. Fischl B. FreeSurfer. Neuroimage 2012;62:774–781 doi: 10.1016/j.neuroimage.2012.01.021.
11. Reuter M, Tisdall MD, Qureshi A, et al. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015 Feb 15;107:107-115. doi: 10.1016/j.neuroimage.2014.12.006.
Figures