1948
Microstructural and functional connectivity changes in the locus coeruleus: a 7T study in cognitively impaired subjects1CHUV, Lausanne, Switzerland, 2UNIGE, Geneve, Switzerland, 3UNIGE, Geneva, Switzerland, 4EPFL, Lausanne, Switzerland, 5CIMEC, Trento, Italy, 6IRCCS Brescia, Brescia, Italy
Synopsis
The locus coeruleus (LC) is a brainstem nucleus thought to undergo early microstructural and functional connectivity changes in Alzheimer’s disease. The in vivo assessment of LC changes is however limited by the resolution of current 3T MRI systems. Here we used high-resolution high-field diffusion weighted imaging (DWI) and resting-state fMRI in cognitively impaired older persons to investigate diffusion and functional connectivity LC changes, and their association with cognition and connectivity of large-scale networks. Results show reduced diffusivity in the caudal LC and associations between microstructural changes, memory deficits, and lower functional connectivity of the dorsal and ventral attention networks.
Introduction
The locus coeruleus (LC) is a brainstem nucleus that regulates cognition, vigilance, and arousal1. LC integrity is associated with memory function in normal aging2,3 and LC disconnection may contribute to memory impairment in Alzheimer’s Disease4 (AD). However, the assessment of LC integrity in-vivo with conventional 3T MRI systems is complicated by the small size of this nucleus (~30-60mm3). However, to date no study has investigated microstructural integrity of the LC in AD, and results of functional connectivity (FC) studies are conflicting5,6,7. Ultra-high field (7T) MRI may overcome these limitations due to higher spatial resolution, signal-to-noise ratio and higher functional contrast-to-noise ratio. In this study, we aimed to assess the microstructural integrity and FC of the LC using diffusion and functional MRI at 7T, in a sample of healthy and cognitively impaired elderly. Moreover, we correlated these measures with cognition and with connectivity of major functional networks.Methods
Participants: 37 elderly individuals (age: 66.1±8.3y, education: 15.6±3.8y, sex: 19 female/18 male, diagnosis: 24 cognitively normal, 13 cognitively impaired) underwent MRI on a 7T Siemens Magnetom scanner (Table 1). All subjects underwent a complete clinical and neuropsychological evaluation, they were dichotomized as follows: cognitively unimpaired (healthy volunteers and individuals with subjective cognitive decline) and cognitively impaired (MCI and dementia patients). A measure of global cognition, MMSE, and two measures of episodic memory, free and cued selective reminding test (FCSRT) immediate and delayed recall, were used for the present investigation.An LC atlas from Ye et al. was used8.
Microstructure: dMRI data were corrected using Gibbs unringing9, topup and EDDY10. Diffusion tensor was estimated using weighted linear least-squares11 and parametric maps for typical metrics (Fractional Anisotropy, FA, Mean Diffusivity, MD; Radial Diffusivity, RD; Axial diffusivity AxD) were calculated. DTI maps were warped into LC atlas MNI space using a combination of rigid, affine and non-linear transformations with Ants (antsRegistrationSyN). Individual anatomical images were used as an intermediate step for the registration. We extracted: mean DTI metrics over the entire LC, over the caudo-rostral LC profile and over the caudal and rostral LC parts separately.
Functional connectivity: Rs-fMRI data pre-processing included MP-PCA denoising12,13, motion correction (FSL), bias field and susceptibility-induced distortions correction with Ants, anatomically-informed spatial smoothing14 (2mm FWHM filter), cleaning with ICA-AROMA (FSL), regression of white matter (WM) and cerebrospinal (CSF) signal, linear detrending and high-pass filtering (0.01Hz). CSF and WM masks were extracted from the anatomical scans with FAST (FSL). The Ye LC atlas and Schaefer 7 networks15 were used as seed for FC analysis. Each seed was warped from MNI to individual native EPI images. Pearsons’ linear correlation between the BOLD signal in the seed and other brain voxels was computed. One-sample tests were used to extract the corresponding connectivity maps (FSL’s randomise). These maps were thresholded and binarized to extract individual FC values for each network and subject.
Multi-modal correlations: We assessed the associations between FC of each network (LC and 7 Schaefer networks), microstructural metrics (FA, MD, RD and AxD in the entire LC and separately by rostral and caudal portions) and cognition (MMSE and FCSRT immediate and delayed recall) using Pearsons’ linear correlation.
Results
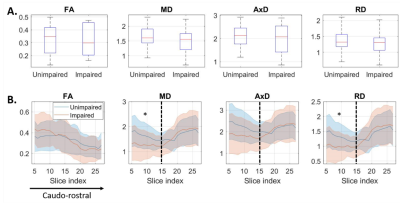
Microstructure: No differences of DTI metrics between impaired and unimpaired subjects were found in the LC as a whole. DTI metric profiles along caudo-rostral LC axis revealed significantly reduced MD and RD in impaired vs healthy subjects in the caudal LC (Figure 1).Functional connectivity: There were no differences between groups in LC connectivity nor in the other networks investigated (default, limbic, frontoparietal, ventral attention, dorsal attention, sensorimotor, visual; p>0.05).
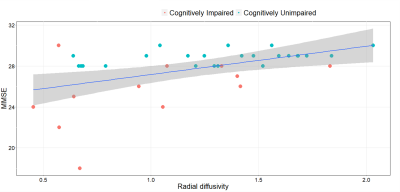
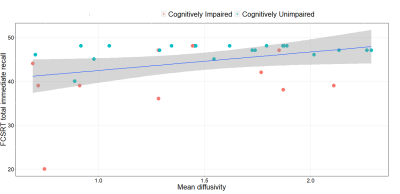
Multi-modal correlations: Diffusivities in the LC were positively correlated to MMSE (MD vs MMSE, r=0.40/p=0.015; AD vs MMSE, r=0.37/p=0.027; RD vs MMSE, r=0.38/p=0.015). This association was observed in the caudal but not rostral portion of the LC for MMSE (MD caudal vs MMSE, r=0.47/=0.004; AD caudal vs MMSE, r=0.40/p=0.015; RD caudal vs MMSE, r=0.48/p=0.003, Figure 2) and episodic memory (MD caudal vs FCSRT total immediate recall, r=0.36/p=0.044; Figure 3). We found no correlation between FC measures and cognitive data, while we observed a positive association between RD, MD in the LC and dorsal attention network (RD vs DAN, r=0.51/p=0.020; MD vs DAN, r=0.56/p=0.009) and salience network (RD vs SAL, r=0.48/p=0.031; MD vs SAL, r=0.55/p=0.011), while AD in the LC was associated with dorsal attention network (AD vs DAN, r=0.55/p=0.012) and sensorimotor network (AD vs SMN, r=0.50/p=0.025) connectivity.
Discussion & Conclusions
In our cohort, microstructure of LC was more sensitive to population differences than FC. However, microstructure results seem to stand out from existing literature, with AD-related degeneration usually reported in the rostral LC. We speculate that lower diffusivity in the impaired group may reflect tau accumulation and/or microglia proliferation16 in the LC, cluttering either the intra- or extracellular spaces or both. In the rostral LC, coexisting microglial proliferation (reduced diffusivity) and neuronal loss (increased diffusivity) may balance each other out and mask the effect. Complementary tau PET imaging will help shed light on these mechanisms. Our results indicate that these microstructural changes may contribute to memory deficits. Finally, associations with functional networks suggest a role of the dorsal and ventral attention systems in mediating these relationships.Acknowledgements
Data for this study were collected at the Centre de la mémoire, Geneva University and University Hospitals, thanks to funds from: Association Suisse pour la Recherche sur l’Alzheimer, Genève; Fondation Segré, Genève; Ivan Pictet, Genève; Fondazione Agusta, Lugano; Fondation Chmielewski, Genève; Velux Stiftung; Swiss National Science Foundation (projects n.320030_182772 and n. 320030_169876); Horizon 2020 (projects n. 667375); Human Brain Project; Innovative Medicines Initiatives (IMI contract n. 115736 and 115952). The authors acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole Polytechnique Fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG). I.O.J. is supported by SNSF grant PCEFP2_194260.References
1. Betts, M. J. et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571 (2019).
2. Dahl, M. J. et al. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat Hum Behav 3, 1203–1214 (2019).
3. Hämmerer, D. et al. Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. PNAS 115, 2228–2233 (2018).
4. Jacobs, H. I. L. et al. Relevance of parahippocampal-locus coeruleus connectivity to memory in early dementia. Neurobiology of Aging 36, 618–626 (2015).
5. Kelberman, M., Keilholz, S. & Weinshenker, D. What’s That (Blue) Spot on my MRI? Multimodal Neuroimaging of the Locus Coeruleus in Neurodegenerative Disease. Frontiers in Neuroscience 14, 1069 (2020).
6. Langley, J., Hussain, S., Flores, J. J., Bennett, I. J. & Hu, X. Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta. Neurobiology of Aging 87, 89–97 (2020).
7. Serra, L. et al. In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer’s disease. Neurobiology of Aging 72, 72–82 (2018).
8. Ye, R. et al. An in vivo probabilistic atlas of the human locus coeruleus at ultra-high field. NeuroImage 225, 117487 (2021).
9. Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine 76, 1574–1581 (2016).
10. Andersson, J. eddy -- a tool for correcting eddy currents and movements in diffusion data. fsl.fmrib.ox.ac.uk/fsl/fslwiki/EDDY. (2013).
11. Veraart, J., Sijbers, J., Sunaert, S., Leemans, A. & Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. NeuroImage 81, 335–46 (2013).
12. Ades-Aron, B. et al. Improved Task-based Functional MRI Language Mapping in Patients with Brain Tumors through Marchenko-Pastur Principal Component Analysis Denoising. Radiology 298, 365–373 (2020).
13. Diao, Y., Yin, T., Gruetter, R. & Jelescu, I. O. PIRACY: An optimized pipeline for functional connectivity analysis in the rat brain. Front. Neurosci. 15, (2021).
14. Huber, L. (Renzo) et al. LayNii: A software suite for layer-fMRI. NeuroImage 237, 118091 (2021).
15. Schaefer, A. et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cerebral Cortex 28, 3095–3114 (2018).
16. Giorgi, F. S. et al. Locus Coeruleus Modulates Neuroinflammation in Parkinsonism and Dementia. International Journal of Molecular Sciences 21, 8630 (2020).Figures