1945
Age-related changes to perivascular and parenchymal fluid flow dynamics with multi-compartment diffusion MRI1USC Mark and Mary Stevens Institute for Neuroimaging and Informatics, University of Southern California, Los Angeles, CA, United States
Synopsis
Perivascular spaces (PVS) play a critical role in fluid transfer and waste clearance in the brain. PVS enlargement is associated with advancing age; however, the functional correlates of these changes are not well understood. Using multi-compartment diffusion models, we assessed the diffusion properties within the PVS and the surrounding parenchyma in a healthy aging cohort. We found age was significantly associated with increased perivascular free water content and reduced parenchymal free water content. These findings provide preliminary evidence of age-related changes to perivascular fluid transfer that may be indicative of waste clearance functional alterations.
Introduction
Perivascular spaces (PVS) are extra-vascular fluid-filled cavities that surround penetrating blood vessels and provide a low resistance pathway for fluid transfer to promote the efficient elimination of waste from the brain [1], [2]. PVS enlargement has been observed in a range of neurological conditions characterized by dysfunctional waste clearance, including Alzheimer’s disease [3], multiple sclerosis [4] and stroke [5]. While commonly considered a biomarker for vascular pathology, recent evidence has also shown that dilated PVS are commonly observed with advancing age [6]; however, the functional significance of such changes in the healthy, cognitively normal population is unclear. Diffusion MRI (dMRI) is a non-invasive neuroimaging method that is sensitive to displacement patterns of diffusing water molecules and can be used to evaluate fluid flow dynamics within and around PVS [7]. Alterations to interstitial fluid diffusion within PVS may be indicative of flow disruptions and diffusion between PVS and the surrounding parenchyma can provide insight into the efficacy of interstitial fluid influx and efflux mechanisms [8]. The goal of this study is to characterize the age-related changes in PVS fluid flow dynamics in healthy aging adults between 35 and 90 years of age using multi-compartment dMRI models to probe tissue compartments characterized by free and restricted diffusion properties. Age-related changes to diffusion metrics were assessed within automatically segmented PVS and in the surrounding parenchyma at variable distances and compared with PVS morphology.Methods
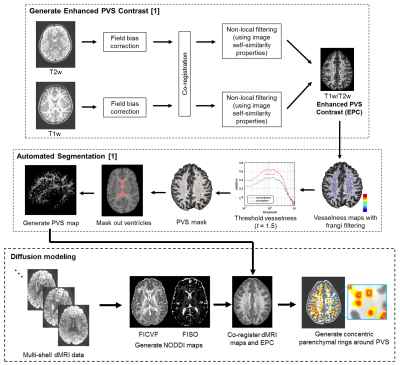
High resolution T1w (voxel size: .8 mm isotropic; FOV: 256x240x166 mm; TR = 2500 ms, TI = 1000 ms, TE = 1.8/3.6/5.4/7.2 ms, FA = 8 degrees), T2w (voxel size: .8 mm isotropic; FOV: 224x224 mm; TR/TE: 3200/565 ms) and multi-shell dMRI scans (b=1500 s/mm2, 3000 s/mm2, 92-93 diffusion-encoding directions per shell, 1.5 mm isotropic voxel, TR=3.23 s) from 575 healthy subjects between 35 and 90 years of age were acquired through the Lifespan Human Connectome Project in Aging. PVS were automatically segmented from enhanced PVS contrasts (EPC) derived from T1w and T2w images using the protocol described in [9] (Figure 1). The NODDI metrics intracellular volume fraction (FICVF) and isotropic volume fraction (FISO) were calculated using the spherical mean technique implemented with the QIT [10]. Diffusion maps were the co-registered to the EPC using a rigid body transform. To assess diffusion metrics as a function of the distance from the PVS, 9 concentric rings were automatically generated from level sets of the PVS boundary with 1 mm spacing between them (Figure 1) and were bounded by the white matter tissue mask to exclude gray matter and ventricles from analyses. Average diffusion metrics were then computed within the PVS masks and the concentric rings in the parenchyma. The main effect of age on PVS diffusion properties were assessed with general linear models, and the age-related changes to parenchymal diffusion properties at variable distances were assessed using linear mixed effects models.Results
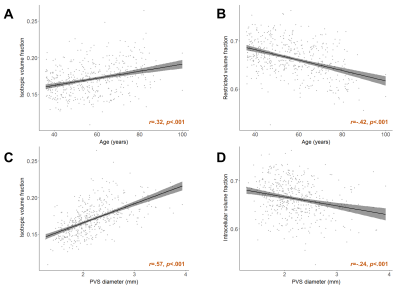
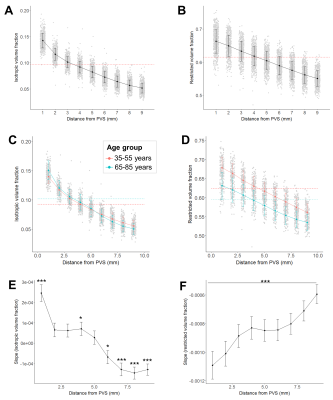
The isotropic volume fraction within segmented PVS was positively associated with age across the lifespan (B=.00048, t(573)=7.31, p<.001, R2=.10) and negatively associated with FICVF (B=-.0011, t(573)=-9.89, p<.001, R2=.17) (Figure 2A-B). The mean PVS diameter in white matter was significantly correlated with the isotropic (B=.026, t(573)=15.14, p<.001) and restricted volume fractions (B=-.019, t(573)=-5.32, p<.001) (Figure 2C-D). The influence of age on FISO and FICVF remained significant after controlling for mean diameter (p<.001). The isotropic volume fraction decreased nonlinearly with distance from the PVS (Figure 3A). When data was stratified into a young (35-55 years) and older (65-85 years) cohorts, a significant interaction between age group and distance from PVS on FISO was observed (F(446,2)=111.4, p<.001) (Figure 3C). FICVF decreased linearly with distance from the PVS (Figure 3B) and a significant interaction between age group and distance from PVS on FICVF was observed (F(446,2)=172.6, p<.001) (Figure 3D). Age-related changes to FISO and FICVF changed with distance from the PVS (Figure 3E-F).Discussion
Our results show age-related differences in perivascular fluid flow that may reflect changes in PVS function with advancing age. The elevated free water content within PVS in older adults may reflect increased fluid flow accommodated by dilated PVS. However, the relationship between PVS free water content and age remained significant after controlling for PVS cross-sectional diameter, which suggests additional mechanisms may contribute to the age-related alterations in perivascular fluid flow. In agreement with [8], we found free water diffusion decreases with distance from PVS. Additionally, we found free water diffusion decreases faster with distance from PVS in older adults compared to younger adults, which may correspond to leakier PVS and less stable fluid homeostasis. Aging is associated with reduced arterial pulsatility and disrupted blood-brain barrier permeability, which can alter interstitial fluid flow and further contribute to the pathogenesis of PVS dilation [11]. It is therefore possible that our findings of altered perivascular fluid flow that accompanies PVS enlargement with advancing age may correspond to changes in waste clearance functionality.Conclusion
Multi-compartment diffusion MRI models enable the quantification of PVS fluid flow dynamics. Our findings demonstrate age-related PVS enlargement is accompanied by alterations to free water dynamics within the PVS and in the surrounding parenchyma and may be indicative of abnormal waste clearance mechanisms.Acknowledgements
The image computing resources provided by the Laboratory of Neuro Imaging Resource (LONIR) at USC are supported in part by National Institutes of Health (grant number P41EB015922). Author KML is supported by the National Institutes of Health (NIH) Institutional Training Grant T32AG058507 and NIH Grant RF1MH123223. Data collection and sharing for this project was funded by the Human Connectome Project.References
[1] J. J. Iliff et al., “A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β,” Sci. Transl. Med., vol. 4, no. 147, 2012.
[2] M. Marín-Padilla and D. S. Knopman, “Developmental aspects of the intracerebral microvasculature and perivascular spaces: Insights into brain response to late-life diseases,” J. Neuropathol. Exp. Neurol., vol. 70, no. 12, pp. 1060–1069, 2011.
[3] G. Banerjee et al., “MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden.,” Brain, vol. 140, no. 4, pp. 1107–1116, Apr. 2017.
[4] R. Conforti et al., “Dilated Virchow-Robin spaces and multiple sclerosis: 3 T magnetic resonance study,” Radiol. Medica, vol. 119, no. 6, pp. 408–414, 2014.
[5] A. Charidimou et al., “Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study,” J. Neurol. Neurosurg. Psychiatry, vol. 84, no. 6, pp. 624–629, 2013.
[6] K. M. Lynch, G. Barisani, A. W. Toga, and F. Sepehrband, “Perivascular space imaging across the lifespan,” in Proc International Society for Magnetic Resonance in Medicine (ISMRM), 2020.
[7] T. Taoka et al., “Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases,” Jpn. J. Radiol., vol. 35, no. 4, pp. 172–178, 2017.
[8] Y. Jiaerken et al., “Dilated perivascular space is related to reduced free-water in surrounding white matter among healthy adults and elderlies but not in patients with severe cerebral small vessel disease,” J. Cereb. Blood Flow Metab., 2021.
[9] F. Sepehrband et al., “Image processing approaches to enhance perivascular space visibility and quantification using MRI,” Sci. Rep., vol. 9, p. 12351, Aug. 2019.
[10] R. P. Cabeen, F. Sepehrband, and A. W. Toga, “Rapid and accurate NODDI parameter estimation with the spherical mean technique,” in Proc International Society for Magnetic Resonance in Medicine (ISMRM), 2019, p. 3363.
[11] J. J. Iliff et al., “Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain,” J. Neurosci., vol. 33, no. 46, pp. 18190–18199, 2013.
[12] A. F. Frangi, W. J. Niessen, K. L. Vincken, and M. A. Viergever, “Multiscale vessel enhancement filtering,” in International Conference on Medical Image Computing and Computer-Assisted Intervention, 1998, pp. 130–137.
Figures