1940
MRI Correlation with Liver Explant Pathology: LIRADS Treatment Response Algorithm for Residual HCC After Y-90 Therapy1Radiology, Mayo Clinic Arizona, Phoenix, AZ, United States, 2Mayo Clinic Alix School of Medicine, Arizona, Scottsdale, AZ, United States, 3Interventional Radiology, Mayo Clinic Arizona, Phoenix, AZ, United States, 4Pathology, Mayo Clinic Arizona, Phoenix, AZ, United States
Synopsis
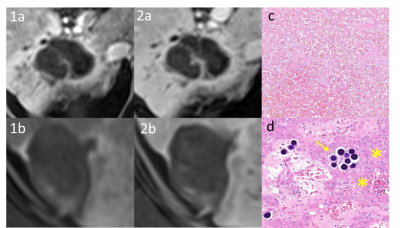
The liver imaging and reporting data system treatment response algorithm (LIRADS TRA) for CT and MRI has been used to predict residual viable tumor after treatment with yttrium-90 (Y-90). This single-center cohort of 20 patients were reviewed using the LIRADS TRA assessing residual viable tumor following Y-90 therapy. Pathology of subsequent liver explants was the reference standard. The LIRADS TRA predicted positive treatment effect (nonviable): NPV 82.4% (95% CI 0.47-1.55), accuracy 75% (72.0-89.4). The results of this preliminary study will need further validation in a larger, similar, pathologic-confirmed cohort.
Abstract
INTRODUCTION:In patients presenting beyond Milan Criteria (MC), hepatocellular carcinoma (HCC) response to locoregional therapy (LRT) is an emerging surrogate marker of tumor aggressiveness that is being used as a better selection criterion for liver transplant (LT). However, optimal protocols and downstaging outcomes are poorly defined. The liver imaging and reporting data system treatment response algorithm (LIRADS TRA)1 for CT and MRI has been used to predict residual viable tumor after treatment with yttrium-90 (Y-90). Yet, there is limited data on how well LIRADS TRA predicts the presence of viable tumor after Y-90 in patients hoping for liver transplant, as prior studies have focused on overall survival after therapy/transplant. This study will assess the efficacy of LIRADS TRA in assessing residual viable tumor following Y-90 therapy, using liver explants as the reference standard.
METHODS:
Single center review of 151 patients who underwent LT and had planning studies for Y-90 therapy. A cohort of 40 HCC patients were identified between 12/2020 and 12/2021. 20 of these had Y-90 treatment for LR-5 observations identified at baseline, had a followup multiphase MRI before transplant (median 34 days before transplant, IQR 18-47), and had followup MRI at least 90 days after Y-90 treatment (median 117 days post treatment, IQR 99.5-186.5).
RESULTS:
There were 18 men and 2 women in the analysis (median age 65; range 52-74). MRI reports were mapped to the LIRADS treatment response algorithm (LR-TR viable, LR-TR nonviable, LR-TR equivocal), and pathology results correlated with the MRI response categories to determine the accuracy of each category. Only 4 of 20 (20%) MRI examinations returned LR-TR equivocal as a result, and none were LR-TR viable. Consequently, a conservative binary MRI result was chosen (LR-TR viable = LR-TR equivocal) as has been previously described.2 The LIRADS TRA predicted positive treatment effect (nonviable): NPV 82.4% (95% CI 0.47-1.55), accuracy 75% (72.0-89.4).
DISCUSSION:
HCC downstaging of patients outside MC has become an important LT strategy. However, assessment of true tumor burden, both prior to and after therapy, is needed to gather accurate comparative data to optimize LT selection schemes. The results of this study suggest that appropriate application of LIRADS TRA for Y-90-treated HCC can provide good accuracy for predicting complete response. Further expansion to include radiology-pathology correlation to treatment response from other modalities (e.g. TACE, DEB-TACE, microwave ablation) in various cirrhotic etiologies will also be beneficial.
CONCLUSION:
Clinicians may be able to draw additional confidence that LR-TR nonviable category at ≥ 90 days post-treatment suggests that residual tumor is unlikely present following Y-90 therapy. However, the results of this preliminary study will need further validation in a larger, similar, pathologic-confirmed cohort.
Acknowledgements
No acknowledgement found.References
1. CT/MRI LI-RADS v2018 | American College of Radiology. Retrieved November 6, 2021, from https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
2. Tae-Hyung Kim, Sungmin Woo, Ijin Joo, Mustafa R. Bashir, Mi-Suk Park, Lauren M. B. Burke, Mishal Mendiratta-Lala Richard K. G. Do. LI-RADS treatment response algorithm for detecting incomplete necrosis in hepatocellular carcinoma after locoregional treatment: a systematic review and meta-analysis using individual patient data. Abdom Radiol 46, 3717–3728 (2021).
3. Toniutto P, Fumolo E, Fornasiere E, Bitetto D. Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review. J Clin Med. 2021 Aug 31;10(17):3932.
4. Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015 Sep;21(9):1142-52. doi: 10.1002/lt.24169. Erratum in: Liver Transpl. 2016 Jan;22(1):138.
5. Degroote H, Piñero F, Costentin C, Notarpaolo A, Boin IF, Boudjema K, Baccaro C, Chagas AL, Bachellier P, Ettorre GM, Poniachik J, Muscari F, Di Benedetto F, Duque SH, Salame E, Cillo U, Gadano A, Vanlemmens C, Fagiuoli S, Rubinstein F, Burra P, Cherqui D, Silva M, Van Vlierberghe H, Duvoux C; French-Italian-Belgium and Latin American collaborative group for HCC and liver transplantation. International study on the outcome of locoregional therapy for liver transplant in hepatocellular carcinoma beyond Milan criteria. JHEP Rep. 2021 Jul
Figures