1934
Magnetic Resonance Spectroscopy in Children and Adolescents with Functional Neurological Disorder
Vishwa Shukla1, Molly Faith Charney1, Sheryl Foster2,3, Wufan Zhao1, Han Sam Jiang1, Kasia Kozlowska4,5,6, and Alexander P Lin1
1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Sydney School of Health Sciences, The University of Sydney, Sydney, Australia, 3Radiology, Westmead Hospital, Westmead, Australia, 4The Children’s Hospital at Westmead Clinical School, Sydney, Australia, 5Psychiatry, Child & Adolescent Health, University of Sydney Medical School, Sydney, Australia, 6Westmead Institute for Medical Research, Westmead, Australia
1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Sydney School of Health Sciences, The University of Sydney, Sydney, Australia, 3Radiology, Westmead Hospital, Westmead, Australia, 4The Children’s Hospital at Westmead Clinical School, Sydney, Australia, 5Psychiatry, Child & Adolescent Health, University of Sydney Medical School, Sydney, Australia, 6Westmead Institute for Medical Research, Westmead, Australia
Synopsis
Functional Neurological Disorder (FND) is characterized by a broad array of neurological symptoms that are thought to reflect aberrant activity in neural networks. This study aims to compare the neurochemical levels in the posterior default mode network (pDMN) in children and adolescents with FND and healthy controls using magnetic resonance spectroscopy (MRS). FND patients exhibited lower levels of tNAA/tCr in the pDMN compared to controls, indicating potential neuronal alterations or modified neuronal-glial signaling.
Introduction
Functional Neurological Disorder (FND) is characterized by a broad array of neurological symptoms—such as loss of motor function/paralysis, abnormal movements, functional seizures, and loss of sensory function—thought to reflect aberrant activity in neural networks1. While these symptoms resolve in most child and adolescent patients, a small subset see their symptoms persist, leading to chronic impairment. There is evidence that FND involves activation of the body's stress system: activation of the brain’s stress system (including an increase in cortical arousal), activation of the Hypothalamus-Pituitary-Adrenal Axis (including higher baseline cortisol), and activation of the autonomic nervous system (including lower resting heart rate variability)2-4. MRS allows for the non-invasive quantification of neurochemicals, which can allow us to gain a better understanding of the pathophysiology underlying FND, which may better inform clinical treatment. The objective of this study is to determine whether children and adolescents with FND have differing neurochemical levels in the pDMN compared to age and sex-matched healthy controls.Methods
19 FND patients aged 8-16 years old and 19 healthy, age and sex-matched controls underwent MRS exam and clinical evaluation. T1 MPRAGE was acquired to visualize brain anatomy and localize the voxel of interest. Short echo, single voxel spectroscopy was acquired in the pDMN (3T, PRESS, TE=30ms , TR=2000ms, 2.5x2.5x4 cm3) (Figure 1). The pDMN was of interest because previous studies using EEG have noted increased baseline activation in the pDMN in FND patients compared to healthy controls4. In addition, altered connectivity in the pDMN has been noted in patients with chronic pain5. Spectra were processed using OpenMRSLab6, which includes channel combination using singular value decomposition, correction of frequency drift, and residual water removal. Myo-inositol (mI), total choline (PCh + GPC), Glutamate + glutamine (Glx), total creatine (Cr + PCr), and total N-acetyl aspartate (NAA + NAAG) levels were quantified using LCModel7 (Figure 2). Two-tailed paired t-tests, with a significance level of <0.05, were used to compare metabolite ratios with tCr between FND patients and sex and age-matched controls.Results
The average age in the FND patients was 13.1 years old and 13.4 years old in the controls. There were 2 male and 17 female participants in each group. There were no issues with data quality (SNR>20, FWHM<0.05ppm, NAA CRLB<5%). The tNAA/tCr ratio was significantly different in the FND patients compared to age and sex-matched controls (p=0.0421). There were lower levels of tNAA/tCr in the pDMN of the FND patients compared to controls (Figure 3). There were no significant differences between the patients and controls for the tCho/tCr, Glx/tCr, and mI/tCr ratios.Discussion
We identified a significant difference in tNAA/tCr in the pDMN between pediatric FND patients and age and sex-matched controls. NAA is found only in neurons and plays a prominent role in axonal-glial signaling, therefore differing levels of NAA could indicate an alteration in this relationship in pediatric FND patients8,9. While lower levels of NAA are often associated with atrophy or neuronal loss, this may not be the case in this population. In a study of participants with PTSD, lower NAA was reported in the hippocampus while hippocampal volumes were comparable between subjects and controls. These findings indicate that lower NAA could represent altered neuronal metabolism or neuronal loss with subsequent compensatory gliosis10. Altered neuronal metabolism, similar to that seen in patients with PTSD, may underly the changes in NAA observed here. Stress has been found to alter both neuronal and glial cells. Although we did not observe changes in mI, a glial marker, associated glial changes should be a focus of future studies. This is only the second study to use MRS to evaluate patients with FND and the first to focus on pediatric patients. Future studies with more participants may allow us to better explore whether neurochemical alterations exist between different clinical subgroups of FND, including those with functional seizures.Acknowledgements
We would like to acknowledge the Center for Clinical Spectroscopy Sundry Funds.References
1. Perez, D. L., Nicholson, T. R., Asadi-Pooya, A. A., et al. Neuroimaging in Functional Neurological Disorder: State of the Field and Research Agenda. NeuroImage Clin. 2021; 30:102623. https://doi.org/10.1016/j.nicl.2021.102623 2. Bakvis P, Spinhoven PE, Giltay J, et al. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia 2009; 51:752-795. 3. Aybek S, Nicholson TR, O'Daly O, Zelaya F, Kanaan RA and David AS. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 2015; 10(4): e0123273. 4. Kozlowska K, Spooner CJ, Palmer DM, et al. “Motoring in idle": The default mode and somatomotor networks are overactive in children and adolescents with functional neurological symptoms. Neuroimage Clin. 2018; 18:730–743. 5. Hemington KS, Rogachov A, Cheng JC, et al. Patients with chronic pain exhibit a complex relationship triad between pain, resilience, and within- and cross-network functional connectivity of the default mode network. Pain. 2018; 159(8):1621-1630. 6. Rowland, B., Sreepada, L., Jiang, S., & Lin, A. (2017). OpenMRSLab: An open-source software repository for Magnetic Resonance Spectroscopy data analysis tools. Paper presented at the European Society of Magnetic Resonance in Medicine and Biology, Barcelona, Spain. 7. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec; 30(6):672-9. 8. Moffett, John R et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in neurobiology 2007; 81(2):89-131. doi:10.1016/j.pneurobio.2006.12.003 9. Baslow, M H. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. Journal of neurochemistry 2000; 5(2):453-9. doi:10.1046/j.1471-4159.2000.0750453.x10. 10. Schuff N, Neylan TC, Lenoci MA, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001; 50(12):952-959. doi:10.1016/s0006-3223(01)01245-8Figures
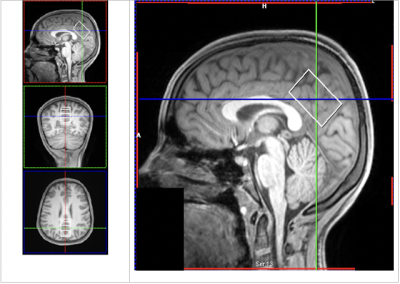
Posterior Default Mode Network (pDMN) Voxel. Example position of pDMN voxel.
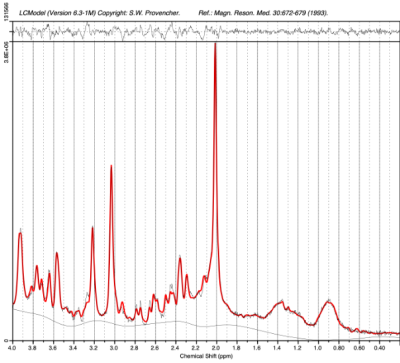
PRESS Spectrum. Example spectrum from pediatric patient with FND acquired in the pDMN with single voxel PRESS MRS and processed with OpenMRS Lab and LCModel.
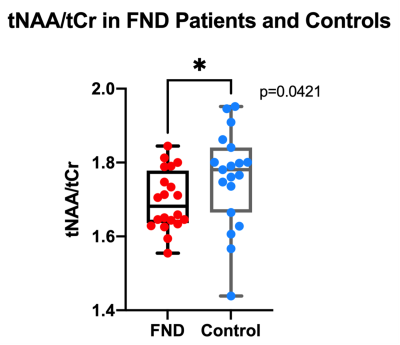
tNAA/tCr in the pDMN. FND patients exhibited lower levels of tNAA/tCr compared to age and sex-matched controls using a two-tailed paired t-test (p=0.0421).
DOI: https://doi.org/10.58530/2022/1934