1929
White matter microstructure characterisation in hippocampal sclerosis and cryptogenic temporal lobe epilepsy1Department of Brain and Behavioral Science, University of Pavia, Pavia, Italy, 2Neuroradiology, I.R.C.C.S. Istituto Neurologico Carlo Besta, Milano, Italy, 3Neuroradiology, IRCCS Mondino Foundation, Pavia, Italy, 4Epilepsy Centre, IRCCS Mondino Foundation, Pavia, Italy, 5“C. Munari” Epilepsy Surgery Centre, ASST Niguarda, Milano, Italy, 6Brain Connectivity Center Research Unit, IRCCS Mondino Foundation, Pavia, Italy, 7NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, UCL Queen Square Institute of Neurology, London, United Kingdom, 8Radiology, IRCCS Policlinico San Donato, San Donato Milanese, Italy
Synopsis
Temporal lobe epilepsy is characterised by heterogeneous aetiology. In this work we focused on hippocampal sclerosis and cryptogenic groups. In order to characterize microstructure white matter alterations underlying the pathology, we performed a regional analysis of the temporal lobe white matter. Our results showed less disruptive microstructural changes in cryptogenic patients. Alterations in Axial Diffusivity and Neurite Density Index in hippocampal sclerosis potentially indicate significant axonal degeneration. Further investigations are needed to ascertain the value of Orientation Dispersion Index as a possible biomarker to predict surgery outcome.
Introduction
In a previous work, we reported specific patterns of white matter (WM) alterations according to the side of the epileptogenic zone in temporal lobe epilepsy (TLE) patients1. Given the heterogeneous nature of the epilepsy presentations, and the availability of surgical specimens on the same dataset, the present work focuses on microstructural characterization of temporal lobe WM in two distinct histopathological sub-groups, defined after surgery: hippocampal sclerosis (HS) and cryptogenic (Crypto) patients. We performed regional-analysis in order (i) to evaluate microstructural abnormalities in relation to the underlying focal aetiology, and (ii) to explore the sensitivity of multiple diffusion metrics to microstructural changes.Methods
Subjects: Fifty-two TLE patients (35.8±10.7yrs, 29 females) and 36 healthy-controls (HC) (32.9±8.4yrs, 17 females) were recruited into a multicenter-study (3TLE)1. Seizures were pre-surgically lateralized according to medical history, neurologic examination, interictal electroencephalography, and positron emission tomography. Subjects underwent surgery, according to their seizure side. A subset of 30 subjects (39.0±8.1yrs, 15 females) were selected for further analysis based on histology of the surgical specimens: 11 were HS (41.8±8.3yrs, 6 females, 5 right) and 19 Crypto (37.4±7.7yrs, 9 females, 15 right); 21 were classified as seizure-free after surgery (Engel=Ia). Of those with worse surgical outcomes (Engel>Ia), 6 belonged to the right-Crypto group.MRI acquisition: Clinical and advanced MRI data were acquired pre-surgery with a 3T Siemens Skyra including double shell diffusion-weighted (DW) images (twice-refocused SE-EPI sequence, TR/TE=8400/93ms, 70 axial-slices, 2.2mm isotropic voxel, 48 non-collinear DW directions/shell, b=1000/2000 s/mm2, 13 volumes with b=0 s/mm2) and a 3DT1-weighted (T1w) image (first echo of a multi-echo FLASH, TR/TE=19/2.46 ms, flip angle 23°, 176 sagittal-slices, 1mm isotropic voxel).
DW-preprocessing: Gibbs-ringing reduction, denoising, eddy-currents and geometrical distortion correction, and motion realignment were performed on DW-images combining commands from the MRtrix32 and FSL3 toolboxes. A diffusion-tensor (DT) model was fitted to the preprocessed DW-images and maps of Fractional Anisotropy (FA), Mean Diffusivity (MD), Axial and Radial Diffusivities (AD, RD) were created. Similarly, the neurite orientation dispersion and density imaging (NODDI) model provided maps of Orientation Dispersion Index (ODI) and Neurite Density Index (NDI). Tract-Based Spatial Statistics (TBSS) was run on the entire 3TLE cohort to identify the WM skeleton1.
Temporal ROI analysis: Voxels of temporal lobe WM were identified by intersecting a mask of each temporal lobe, extracted from a brain atlas in MNI-space4, and the TBSS skeleton previously calculated. For each temporal ROI, mean values of DT and NODDI-derived metrics were extracted. In addition, the hippocampi were segmented from the volumetric (3DT1) scan using FIRST(FSL) and their volumes calculated.
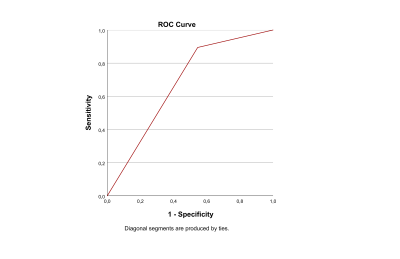
Statistics: Statistical tests were performed using SPSS21(IBM). General linear models were built to compare diffusion-derived metrics of the temporal lobes between groups. In HC vs HS patients and HC vs Crypto patients age, gender, laterality of pathology and hippocampal volume were used as covariate. In HS vs Crypto, diffusion-derived metrics of the pathological or non-pathological temporal lobe were compared, using age and gender as covariate. For all comparisons, a two-sided p<0.05 was considered significant. A further sub-analysis was performed on the Crypto patients (with right TLE), as this was found to be the histological group with a higher proportion of not resolved seizures (40%). HC, seizure-free-Crypto and other-Crypto were compared using age and gender as covariates. A discriminant analysis was performed using HS and Crypto as the dependent variable and microstructural metrics as independent variables; receiving operating characteristics (ROC) curves were created to visualize and assess the sensitivity and specificity of the analysis.
Results
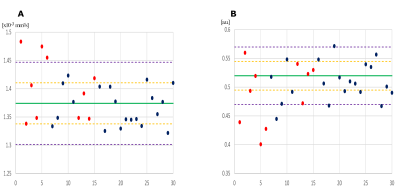
Compared to HCs, HS patients showed lower FA and NDI, higher MD, AD and RD in the left temporal lobe, while Crypto patients showed only MD and RD reductions in the left temporal lobe (Figure-1).Compared to Crypto, HS patients showed higher AD (p=0.026) and lower NDI (p=0.041), in the affected temporal lobe (Figure-2). The best discriminant power obtained (66.7%) including all metrics was reached using NDI. To visualize these results, ROC curves are reported in Figure-3.
Compared with HC, the Right other-Crypto patients showed significantly lower FA (p=0.035), higher ODI (p=0.036) in the right temporal lobe and higher ODI (p=0.003) in the left temporal lobe, whereas no such differences were seen for the Right seizure-free-Crypto patients.
Discussion and Conclusion
Our analysis indicates that HS patients are characterised by microstructural alterations of the WM of the left temporal lobe, corrected for epilepsy lateralisation, captured by all DT metrics plus NDI. Crypto patients, on the other hand, presented alterations only of MD and RD metrics, hence they seem to be characterised by less disruptive microstructure changes compared to HC. Moreover, comparing metrics measured in the affected temporal lobe WM, differences were found between HS and Crypto patients when considering AD and NDI, potentially indicating significant axonal degeneration. Use of NDI metrics of the affected temporal lobe as classifier showed a moderate accuracy for distinguishing Crypto from HS patients, with high accuracy but low sensitivity.The results in the Crypto group sub-analysis found that Crypto patients who were not cured by surgery to the affected temporal lobe presented ODI alterations of the temporal lobe that was considered clinically non pathological. It will be important to assess whether ODI may be a possible biomarker that could predict surgery outcome.
Acknowledgements
Data were collected within the 3TLE multicentric research project (Italian Ministry of Health, NET-2013-02355313). ED and FP receive funding from H2020 Research and Innovation Action Grants Human Brain Project (#785907, SGA2 and #945539, SGA3). ED receives funding from the MNL Project “Local Neuronal Microcircuits” of the Centro Fermi (Rome, Italy). CGWK receives funding from the MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541), BRC (#BRC704/CAP/CGW), MRC (#MR/S026088/1). CGWK is a shareholder in Queen Square Analytics LtdReferences
1) N. Rolandi et al., “White matter microstructure characterisation in left and right temporal lobe epilepsy (TLE) using TBSS,” Proc Int Soc Magn Reson Med, vol. 25, no. 3, pp. 460–464, 2020, doi: 10.1111/jon.12154.
2) J. D. Tournier et al., “MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation,” NeuroImage, vol. 202. Academic Press Inc., Nov. 15, 2019, doi: 10.1016/j.neuroimage.2019.116137.
3) M. Jenkinson, C. F. Beckmann, T. E. J. Behrens, M. W. Woolrich, and S. M. Smith, “FSL,” Neuroimage, vol. 62, pp. 782–790, 2012, doi: 10.1016/j.neuroimage.2011.09.015.
4) R. Toro et al., “Brain volumes and Val66Met polymorphism of the BDNF gene: Local or global effects?,” Brain Struct. Funct., vol. 213, no. 6, pp. 501–509, 2009, doi: 10.1007/s00429-009-0203-y.
Figures
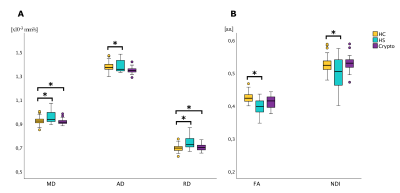
Figure 1: Boxplot of the DW-parameters in left temporal lobe ROI. Significant differences (p<0.05) between groups are marked with an asterisk.
HC: Healthy controls; HS: Hippocampal sclerosis; Crypto: cryptogenic epilepsy; MD: Mean Diffusivity; AD: Axial Diffusivity; RD: Radial Diffusivity; FA: Fractional Anisotropy; NDI: Neurite Density Index.