1926
Segmentation and Quantification of Venous structures and Perivascular Spaces in the Thalamus in Epilepsy Patients at 7T1Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Icahn School of Medicine, Graduate School of Biomedical Sciences, New York, NY, United States, 3Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Department of Neurology, Mount Sinai Hospital, New York, NY, United States, 5Department of Computer Science, Math, Physics, and Statistics, University of British Columbia, Vancouver, BC, Canada
Synopsis
Here we outline a preliminary analysis using a novel method which leverages UHF neuroimaging to measure detectable differences in vasculature within the thalamus that may not be detectable at lower field strengths. We provide a tool for detection and quanitifcation of vessels, perivascular spaces, and subsequent overlaps within the thalamus which may be relevant to uncover possible underlying neuroinflammatory processes in focal epilepsy patients. In our analysis, we found a significant difference in the number of thalamic vessels in patients compared to controls, providing a possible marker to measure abnormal or disordered vessel growth possibly associated with increased seizure activity.
Introduction
Epilepsy is a neurological disorder that affects 50 million people worldwide. Approximately ⅓ of epileptic patients do not respond to anti-seizure medications. Due to different seizure subtypes and the complexity of the disorder, it is particularly challenging to determine a seizure origin. Persistent seizures may be correlated with network changes in the brain and the thalamus is thought to be one of the final common pathways for epilepsy, meaning that microstructural or vascular changes could contribute to changes or be a result of focal seizure networks. These changes could be indicative of larger alterations involving neuronal vasculature and neuroinflammatory processes linked to glymphatic drainage. In order to visualize possible vascular abnormalities within the thalamus, integration of the high sensitivity and enhanced contrast achieved through the use of ultra-high field (UHF) MRI, may be useful to identify abnormalities not detectable at lower field strengths, such as PVS. Perivascular spaces (PVS) are small fluid-filled spaces in the brain that are thought to be involved in glymphatic clearance. The presence of these structures have been associated with neuroinflammation and may be a byproduct of seizure activity or dysfunction in vasculature or drainage. Segmentation and quantification of vessels, PVS, and the overlap of PVSs and vessels in the thalamus may provide insight into the role of glymphatic drainage, the thalami, and overall networks involved in epilepsy. In this study, we performed an exploratory analysis using a novel technique to quantify PVS in the thalamus, quantify vessels, and overlaps, in efforts to explore vascular differences in patients with focal epilepsy relative to healthy controls (HC).Methods
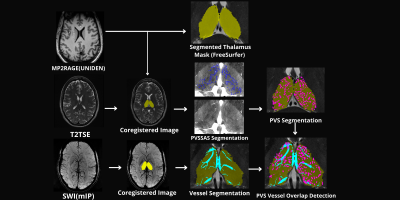
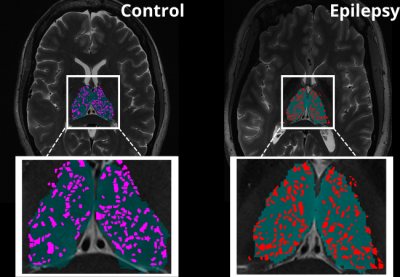
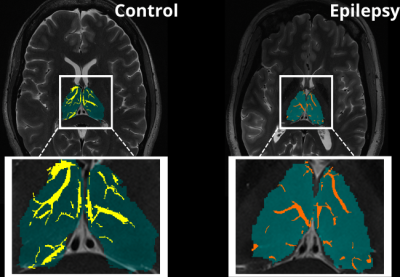
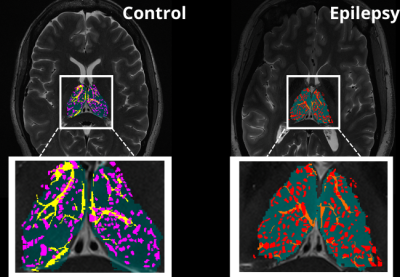
An exploratory analysis was performed in ten subjects. Five epilepsy patients with a primary diagnosis of mesial temporal lobe epilepsy (MTLE) or focal neocortical epilepsy were age and sex matched with five HC participants who had no current or lifetime history of seizure or epilepsy. Each subject underwent a 7T MRI (Magnetom, Siemens, Erlangen) scan which included a T1-weighted MP2RAGE35 (UNIDEN) sequence (TA 7:26; voxel size 0.8 × 0.8 × 0.8 mm3; FOV 255 × 183 mm2; TR 6000 ms; TE 5.1 ms; TI1/TI2 1050/3000 ms; FA1/FA2 5/4; Matrix 282 × 146; BW 130 Hz/pixel; iPAT 3), an SWI sequence (TA 7:30; voxel size 0.2 × 0.2 × 1.5 mm3; TR 23 ms; TE 14 ms; FA 12; matrix 1024 × 832; BW 150 Hz/Pixel; iPAT 3), an Axial T2-weighted turbo spin echo (T2TSE) sequence (TR = 6000 ms, TE = 69 ms, flip angle = 150°, Field of View = 202 x 185 mm2, matrix = 512x464, in-plane resolution 0.4 x 0.4 mm2, slice thickness = 2 mm, slices = 40, BW = 279 Hz/pixel, time = 6:50 min). All UNIDEN images were run through recon-all using FreeSurfer 7.2, and thalamic segmentation was performed. All subsequent thalamus masks and the FreeSurfer generated T1 image were coregistered to the T2TSE image and the UNIDEN image and thalamus masks were coregistered to the SWI mIP image using SPM 12 (Figure 1). Following coregistration, the T2TSE and segmentation of the thalamus were used to detect PVS using a Frangi-based detection tool called PVSSAS which was optimized for detection of PVS in the gray matter implemented in Matlab2021b (Figure 2). Due to partial volume effects, some PVS may be combined if within one voxel of one another. The SWI mIP images were processed also with the segmentation of the thalamus using a Frangi-based detection and segmentation tool implemented in Matlab(Figure 3). The Vessel mask was coregistered to the T2TSE. The resulting coregistered vessel mask and PVS mask were used to quantify the number of vessels, PVS, overlaps, and number of PVS overlaps per vessel detected, using a Hessian detection filter linked on an 18-connected network (Figure 4). Statistical analysis was performed using R. All data were tested for normality using Shapiro Wilks testing, and Student’s t-test was used, to determine differences in total number of PVS, vessels, overlaps, vessels with more than one PVS, and what percentage of PVS were perivenular, between groups.Results
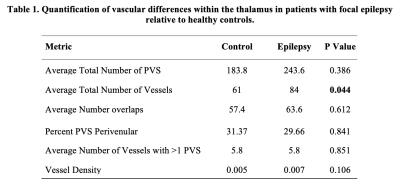
When assessing differences in the total number of PVS, overlaps, vessels with more than one PVS, vessel density, and what percentage of PVS were perivenular, there were no significant differences between groups (Table 1). We found significant differences in the number of vessels in epilepsy patients compared to controls (P> 0.044).Discussion
In this exploratory analysis, we developed a process for the quantification and detection of vessels and other neuroinflammatory markers within the thalamus that may be important in the pathophysiology of epilepsy. We found a significant difference in the number of thalamic vessels in patients compared to controls. This increase in vessel number within epilepsy patients could possibly be explained by an increase in branching and be used to measure integrity of vasculature in this region. Here we have provided a metric that may be useful to measure abnormal or disordered vessel growth that could be associated with or be a byproduct of increased seizure activity.Conclusion
This method can potentially provide a tool which leverages UHF neuroimaging to measure detectable differences in vasculature and uncover possible underlying neuroinflammatory processes in patients with focal epilepsy.Acknowledgements
This work was supported by the National Institutes of Health - National Institute of Neurological Disorders and Stroke - R00 NS070821, www.ninds.nih.gov/ (PB); Icahn School of Medicine Capital Campaign (PB); Translational and Molecular Imaging Institute (PB); Department of Radiology, Icahn School of Medicine at Mount Sinai (PB).References
World Health Organization. (2019). Epilepsy: a public health imperative. World Health Organization.
Kwan, P., & Brodie, M. J. (2000). Early identification of refractory epilepsy. New England Journal of Medicine, 342(5), 314-319.
Feldman, R. E., Rutland, J. W., Fields, M. C., Marcuse, L. V., Pawha, P. S., Delman, B. N., & Balchandani, P. (2018). Quantification of perivascular spaces at 7T: A potential MRI biomarker for epilepsy. Seizure, 54, 11–18. https://doi.org/10.1016/j.seizure.2017.11.004
Feldman, R. E., Marcuse, L. V., Verma, G., Brown, S., Rus, A., Rutland, J. W., Delman, B. N., Balchandani, P., & Fields, M. C. (2020). Seven-tesla susceptibility-weighted analysis of hippocampal venous structures: Application to magnetic-resonance-normal focal epilepsy. Epilepsia, 61(2), 287–296. https://doi.org/10.1111/epi.16433
Mestre, H., Kostrikov, S., Mehta, R. I., & Nedergaard, M. (2017). Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clinical science (London, England : 1979), 131(17), 2257–2274. https://doi.org/10.1042/CS20160381A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology.
Iglesias, J.E., Insausti, R., Lerma-Usabiaga, G., Bocchetta, M., Van Leemput, K., Greve, D., van der Kouwe, A., Caballero-Gaudes, C., Paz-Alonso, P. Neuroimage (accepted).
Penny, W. D., Friston, K. J., Ashburner, J. T., Kiebel, S. J., & Nichols, T. E. (Eds.). (2011). Statistical parametric mapping: the analysis of functional brain images. Elsevier.
Figures