1925
Microstructural Reorganizations in Thalamic Nuclei Across Characteristics of Temporal Lobe Epilepsy1Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
Temporal lobe epilepsy (TLE) is the most common form of epilepsy which can become medically refractory and referred for surgery. The thalamus has been shown to demonstrate both structural and functional changes in TLE. However, little is known about microstructural alterations within individual thalamic nuclei. In this study, we used diffusion tensor imaging (DTI) to determine if subjects with unilateral TLE demonstrate hemispheric and intra-hemispheric differences in thalamic nuclei across clinical characteristics. We report statistically significant differences in nuclei in subjects with tonic-clonic seizures and mesial temporal sclerosis in both hemispheric and intra-hemispheric comparisons.
Introduction
Temporal Lobe Epilepsy (TLE) is the most common form of epilepsy to become medically refractory and be referred for surgical intervention1. Although the exact pathophysiology of epilepsy is still uncertain, it is established that TLE can lead to structural and functional changes to the brain. In particular, the thalamus is a subcortical structure that serves as the brain’s primary sensory relay via numerous connections to the sensory cortex. Certain patterns of structural and functional changes in the thalamus have been observed with TLE2-4, and studies using diffusion imaging have found changes in diffusion scalars in the thalamus such as in thalamocortical tracts or associated white matter tracts5,6. ROI-based studies have also demonstrated diffusional changes in the thalamus7,8. To our knowledge, few studies have investigated changes in diffusion tensor metrics in individual nuclei due to TLE. In this study, we use diffusion tensor imaging to characterize microstructural alterations in thalamic nuclei based on hemispheric differences as well as intra-hemispheric differences across clinical factors such as seizure outcomes, and the presence of mesial temporal lobe sclerosis (MTS) and tonic-clonic (TC) seizures in subjects with unilateral TLE.Methods
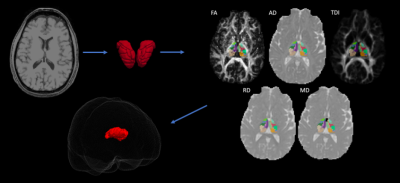
We conducted a retrospective study of 23 subjects with unilateral TLE that underwent surgical intervention ranging from temporal lobectomy, laser ablation, or sEEG followed by surgical intervention. Outcomes based on seizure free status were obtained for each patient following at least 1 year post operation. Of the 23 subjects, 11 were seizure free at least one year following surgery and 15 had MTS. Preoperative 3T T1 and DTI scans were acquired for each subject which were used for analysis. Patient diffusion data was corrected for motion artifacts and eddy current distortions. Then, diffusion tensor estimates per voxel were fit for each patient scan to generate the fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) maps. In addition, whole brain tractography was performed using MRTRIX to acquire tract density measures (TDI). To acquire thalamic ROIs, Freesurfer9 was utilized to automatically segment thalamic nuclei for each patient. The thalamic nuclei segmentations were overlayed to the patient diffusion space to acquire DTI metrics FA, AD, RD, and MD, and tract density (TDI). Figure 5 provides a brief overview of the steps involved. Thalamic nuclei were compared hemispherically between the contralateral and ipsilateral (to the epileptogenic foci) sides as well as intra-hemispherically across clinical factors like seizure outcomes and presence of MTS and TC seizures in both ipsilateral and contralateral hemispheres. Statistical analysis was performed via a multiple Mann-Whitney U-test with significance threshold at p < 0.05.Results
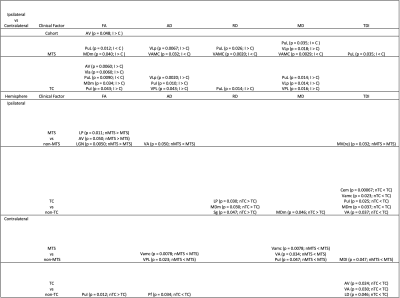
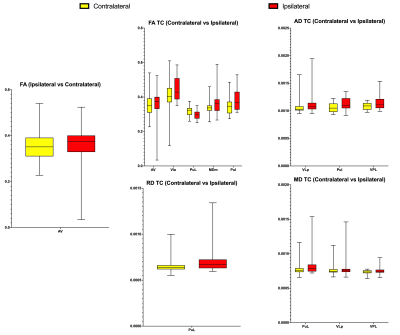
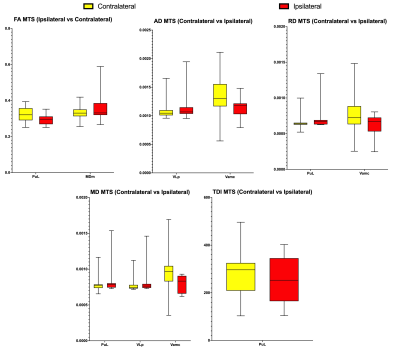
Hemispheric comparison with all subjects demonstrated a statistically significant difference in mean FA in the Anteroventral nucleus. Other hemispheric comparisons in subjects with MTS and TC also yielded numerous thalamic nuclei with significant differences across various DTI metrics and TDI. Additionally, comparison of the same hemisphere across the presence of MTS and TC was also performed which yielded significant differences in thalamic nuclei belonging to both the ipsilateral and contralateral sides. However, comparison involving seizure outcomes did not yield any significant differences. Figure 1 displays significant thalamic nuclei and Figure 2 displays the abbreviations used for thalamic nuclei. Figures 3 and 4 display graphs of statistically significant nuclei across hemispheric comparisons.Discussion
Our results indicate the presence of alterations in the microstructure of thalamic nuclei in subjects with TLE between hemispheres and within hemispheres across clinical factors like MTS and the presence of TC seizures. Changes in the DTI scalars FA, AD, RD, and MD, and TDI may indicate a variety of processes such as change in myelination status or gliosis10. These changes based on diffusion scalars, however, are sensitive but not specific to any given process such as chronic neurodegeneration or neuroplasticity due to TLE. Nonetheless, based on literature, the causes for alterations in diffusion metrics can be surmised. Decreased FA, which indicates decreased anisotropic diffusivity, could suggest white matter tract alterations due to axon damage, demyelination, and fiber loss. RD and AD reflect axonal density and integrity respectively11. Thus, the observed increases in both RD and AD could reflect altered myelin parameters.Conclusion
DTI metrics may serve as a sensitive imaging biomarker to detect potential pathological processes in the brain due to TLE. The detection of microstructural alterations within individual thalamic nuclei may provide further insight into the thalamus’ role in the pathogenesis of epilepsy in addition to further elucidating any clinical implications that may reflect microstructural changes in the thalamus. While the results only demonstrated changes in a few nuclei, these results are preliminary and warrant continued study with a larger population.Acknowledgements
No acknowledgement found.References
1. Asadi-Pooya AA, Stewart GR, Abrams DJ, Sharan A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg. 2017;99:662-666. doi:10.1016/j.wneu.2016.12.074
2. Park KM, Kim TH, Mun CW, et al. Reduction of ipsilateral thalamic volume in temporal lobe epilepsy with hippocampal sclerosis. J Clin Neurosci. 2018;55:76-81. doi:10.1016/j.jocn.2018.06.025
3. Chang Y-HA, Marshall A, Bahrami N, et al. Differential sensitivity of structural, diffusion, and resting-state functional MRI for detecting brain alterations and verbal memory impairment in temporal lobe epilepsy. Epilepsia. 2019;60(5):935-947. doi:10.1111/epi.14736
4. Li R, Zhang L, Guo D, et al. Temporal lobe epilepsy shows distinct functional connectivity patterns in different thalamic nuclei. Brain Connect. 2021;11(2):119-131. doi:10.1089/brain.2020.0826
5. Zhang Y, Jiang L, Zhang D, et al. Thalamocortical structural connectivity abnormalities in drug-resistant generalized epilepsy: A diffusion tensor imaging study. Brain Res. 2020;1727:146558. doi:10.1016/j.brainres.2019.146558
6. Chen C, Li H, Ding F, et al. Alterations in the hippocampal-thalamic pathway underlying secondarily generalized tonic-clonic seizures in mesial temporal lobe epilepsy: A diffusion tensor imaging study. Epilepsia. 2019;60(1):121-130. doi:10.1111/epi.14614
7. Gong G, Concha L, Beaulieu C, Gross DW. Thalamic diffusion and volumetry in temporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Res. 2008;80(2-3):184-193. doi:10.1016/j.eplepsyres.2008.04.002
8. Kim CH, Koo B-B, Chung CK, Lee J-M, Kim JS, Lee SK. Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: a diffusion tensor imaging study. Epilepsy Res. 2010;90(1-2):21-27. doi:10.1016/j.eplepsyres.2010.03.0029.
9. Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. 2018;183:314-326.doi:10.1016/j.neuroimage.2018.08.012
10. Bellani M, Boschello F, Delvecchio G, et al. DTI and myelin plasticity in bipolar disorder: integrating neuroimaging and neuropathological findings. Front Psychiatry. 2016;7:21. doi:10.3389/fpsyt.2016.0002Müller H-P, Roselli F, Rasche V, Kassubek J. Diffusion Tensor Imaging-Based Studies at the Group-Level Applied to Animal Models of Neurodegenerative Diseases. Front Neurosci. 2020;14:734. doi:10.3389/fnins.2020.00734
11. Müller H-P, Roselli F, Rasche V, Kassubek J. Diffusion Tensor Imaging-Based Studies at the Group-Level Applied to Animal Models of Neurodegenerative Diseases. Front Neurosci. 2020;14:734. doi:10.3389/fnins.2020.00734
Figures