1915
High Resolution Postmortem Brain Imaging at 7 Tesla using Parallel Transmission and a Simple Set-Up1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2Institute for Neuroscience and Medicine, INM-1, Structural and Functional Organisation of the Brain, Forschungszentrum Juelich GmbH, Juelich, Germany, 3University of Paris-Saclay, CEA, CNRS, BAOBAB, NeuroSpin, Gif sur Yvette, Paris-Saclay, France, 4Siemens Healthcare SAS, Saint-Denis, France, 5Cecile and Oskar Vogt Institute for Brain Research, Heinrich Heine University Duesseldorf, University Hospital, Duesseldorf, Germany, 6Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
In this work we present a simple set-up for high resolution postmortem imaging at ultra high field strength. The processed images have high, as well as homogeneous gray/white matter contrast over the whole brain. Additionally, during post-processing, the background signal of the fixation liquid is almost completely removed, allowing for an optimal image registration process after histological sectioning.
Purpose
High-resolution postmortem brain scans provide reslicable images of the brain tissue before permanent sectioning [1,2] and facilitate the visualizing of the microstructural neuroanatomy of the human brain. The histological processing inevitably introduces artifacts such as distortion and tearing of the brain tissue. This in turn introduces problems during the 3D reconstruction process. However, the histological sections can be nonlinear registered to an MRI, which serves as an undistorted frame of reference.To achieve an accurate registration result, the MR data has to have homogeneous and high gray/white matter contrast over the whole brain volume, and as less as possible background signal to aid the registration process.
In this work, we describe a relatively simple experimental set-up for acquisition of such high resolution, high contrast, ex vivo brain images utilizing a 7T MR system with parallel transmission hardware.
Methods
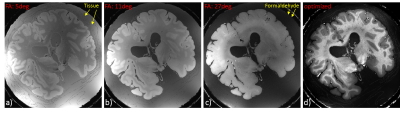
The brain was placed in a custom-made coil shaped container filled with degassed 4% phosphat-buffered formaldehyde solution, and exposed to 500 mbar vacuum for 12 h (20 deg Celsius) to remove residual air bubbles. Figure 1 shows and describes the straightforward brain container assembly. All parts were obtained from an ordinary supply store.Imaging was performed on a whole-body investigational MAGNETOM 7T scanner (Siemens AG, Healthineers, Erlangen, Germany). Image homogenization was achieved by employing tailored, non-selective kT-points pulses (15 sub-pulses) [3]. The data were acquired using a head array coil with 32 receive and 8 transmit channels (Nova Medical, Wilmington, USA). To remove residual bias fields as well as unwanted background signal, T1 quantification was performed on multi flip angle gradient echo images. The flip angle sampling was as follows: Minimum/maximum flip angle according to Olsson et. al. [4] (5 and 29 deg), Ernst angle (11 deg), and two additional flip angles in between (7 and 19 deg) , TR/TE: 20/5 ms, 200um isotropic resolution, 11h scan time per flip angle. The residual transmit field bias of the kt pulse was removed by flip angle mapping using an additional AFI measurement [5] : 5 mm isotropic resolution, 1 min scan time.
The data were fitted to a modified Ernst equation [6], and images with optimized gray/white matter contrast were computed as follows:
Iopt = C * R1/ M0
with longitudinal relaxation rate R1, M0 equilibrium magnetization, and a global scaling factor C. Finally, N4 bias field correction and image denoising was applied [7].
Results
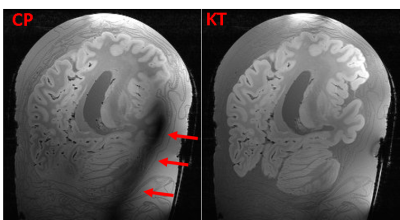
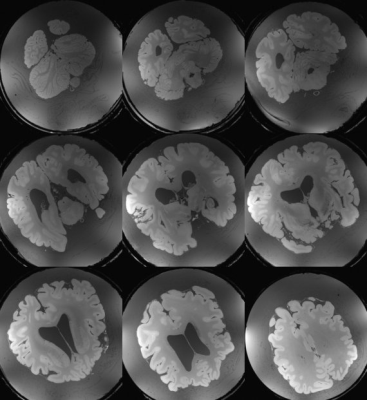
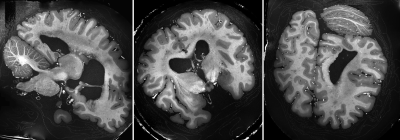
In Figure 2 a comparison between a CP mode and full pTX acquisition is shown. Severe signal drop-outs appear in the brain when employing CP mode (Siemens Trueform) for imaging. In contrast, applying kt-points mitigates the B0 and B1 field inhomogeneities and removes those drop-outs completely over the whole brain volume (Figure 2 right, Figure 3).Fitting the multi flip angle data to the modified Ernst equation and applying equation 1 enhances the gray/white matter contrast and removes most of the background signal (Figure 4). In Figure 5 representative slices after bias field correction and image denoising on the optimized contrast image are shown. Anatomical details are visualized in great detail.
Discussion
In this work and easy to implement workflow for whole brain postmortem imaging on a 7T MR scanner is presented. Besides parallel transmission hardware, no specialized equipment such as dedicated RF coils are needed. Furthermore, a complicated and expensive fixation procedure of the postmortem brain (i.e. proton-free oil to eliminate background signal) is rendered unnecessary.Next, besides increasing the image resolution, multi parameter mapping [8] will be implemented to gain further neuroanatomic insights for comparison with histology data.
Acknowledgements
No acknowledgement found.References
[1] Amunts K. et al.: Science 2013, 340
[2] Amunts et al., Science 2020; 369:988–992
[3] Cloos M. A. et al.: MRM 2012; 67:72–80
[4] Olsson et al.: MRM 2020; 84:1347–1358
[5] Yarnykh et al.: MRM 2007; 57:192-200
[6] Chang et al.: MRM 2008; 60:496-501
[7] ANTS toolbox: Available at: http://stnava.github.io/ANTs
[8] Weiskopf et al.:Front Neurosci. 2013; 7:95
Figures