1914
Exploring Ultra-High Resolution Imaging of the Ex Vivo Whole Brain: Initial Results with Balanced Steady State Free Precession Sequences at 3T1Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engineering, Faculty of Medicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurological Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland, 3Dept. of Radiology, Division of Radiological Physics, University Hospital Basel, Basel, Switzerland, 4Department of Cognitive Neurology, MR-Research in Neurology and Psychiatry, University Medical Center Göttingen, Göttingen, Germany, 5Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany
Synopsis
Balanced steady state free precession (bSSFP) sequences provide the highest signal intensity per unti time, which makes them virtually predestined for ultra-high resolution MR imaging at 3T. Their sensitivity to susceptibility effects and demand for high performance, however, represent two major drawbacks. It will be shown that a carefully chosen protocol, which also includes common phase cycling techniques, will enable artifact-free 200-microns isotropic bSSFP acquisitions of the entire human fixed brain within approximately 27h.
Introduction
Ultra-high resolution imaging (URI) of the entire human ex vivo brain has become very popular lately 1-5. The possibility to use extended acquisition times under ex vivo conditions leads to impressive MR images that are valuable for both clinical and scientific research. Recent publications were able to achieve isotropic resolutions up to 100µm (7T) and 160µm (3T), respectively, making use of T2* weighted RF spoiled gradient echo (FLASH) sequences with very low receiver bandwidth 4,5. Such FLASH sequences have the advantage to provide a relatively high SNR, impose a low demand on the MR system hardware, and also demonstrate a very good long term stability during measurement 5.Yet, FLASH sequences, belonging to the class of steady state MR sequences – being the methods of choice under ex vivo conditions 5 – are still inferior in terms of signal acquisition efficiency compared to the balanced steady state free precession (bSSFP) sequences. In fact, Pefferbaum et al. already presented bSSFP imaging with a very high 200µm in-plane resolution but thick 1mm slices on an isolated cerebellum specimen 1; and Tendler, Miller and co-authors perform isotropic 330µm bSSFP imaging including phase cycling as a standard for ex vivo examinations 2,3.
In this work, we present initial results regarding our investigation of the viable boundaries of a sustainable, very-high isotropic spatial resolution bSSFP based MRI approach, dedicated for whole-brain ex vivo acquisitions on a standard 3T MR system.
Methods
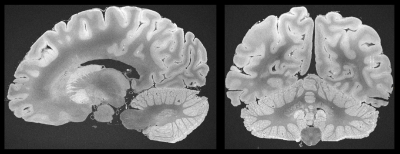
The brain of a patient with MS was fixed in 4% formalin 24h after death. For MRI acquisition, the brain was positioned in a dome-shaped container as depicted in 6-8 and immersed in a fluorinated oil (Fomblin). Air bubbles were aspirated through the spout of the container through a vacuum pump. All acquisitions were performed with a 3T whole-body MR system using a standard 20-channel head coil.In a pre-experiment fast T1 maps were acquired according to 9, T2 maps were fit based on single spin echo acquisitions repeated five times with the different TE={8,16,24,32,40}ms. As a result, based on published theory 10, the optimal bSSFP flip angle was determined accordingly.
For bSSFP based URI, an in-house bSSFP sequence was programmed that circumvents typical restrictions like maximal 3D matrix size and that can exploit the MR system’s full hardware performance on demand.
In another pre-experiment, test acquisitions with fast bSSFP sequences of reduced 400µm isotropic resolution were conducted using different excitation flip angles of {30°,45°,60°,75°,90°}. The residual MR parameters were very similar to the following URI variant of full (200μm)3 isotropic resolution: matrix 960x784x640, transverse slices with readout in A-P direction, TR/TE=13.0ms/6.5ms, bandwidth=290Hz/Px, flip angle 80°, four different phase cyclings of {0°,90°,180°,270°} were applied 11, TAbase=01:48:51h, altogether 15 repetitions were measured for later averaging (TAtotal=27.5h). This URI protocol could be sustained continuously by our MR system over a long period of time (cf. Discussion).
Results
Figure 1 shows a contrasting juxtaposition of the flip angle test sequences. For our experiments, the optimal flip angle was ca. 45° to 50° 10, however, we decided using 80° instead to introduce a stronger T2 sensitivity. The resulting signal loss of a few percent was tolerated accordingly. Figure 2 investigates the (changing) behavior of the field inhomogeneity induced dark band artifacts. As a result, Figure 3 depicts the mean images for the different applied phase cyclings. The resulting combined (final) image demonstrates a considerably improved SNR and homogeneity (Figure 3).Figure 4 shows examples of reformations underlining the feasibility of the used bSSFP approach.
Discussion and Conclusion
Based on their acquisition efficiency, bSSFP sequences are predestined for ex vivo URI applications. Two drawbacks, however, are the banding artifacts and the demand for high performance regarding the gradient and RF system. Miller et al and Tendler et al. observed that the banding artifacts can be tackled by using common phase cycling schemes for their 330μm acquisitions 2,3. Indeed, our initial results confirm that this observation is still true even for our 200μm acquisitions, despite the “long TR” of 13ms and despite of additional drift effects over the long scan times.The high performance demand posed a challenge, however, and eventually led to a mandatory reduction of the overall sequence performance: An initial 170μm protocol was not sustainable and stopped the MR system after a few minutes (entire gradient system failure - a reproducible error). It could be argued though that a radial k-space sampling scheme would be less gradient demanding overall, yet, such a methodology would introduce other challenges for reconstruction and stability.
Moreover, a different 180μm protocol was sustainable overnight but lead to a serious helium boil-off in the MR system. Consequently, and for the moment, the isotropic resolution was reduced to 200μm, the overall gradient performance to 90% of maximum, and at last, RF pulses of longer 2.56ms duration were applied to reduce the permanent strain on the RF system as well.
To conclude, based on their acquisition efficiency, bSSFP sequences represent an interesting and valuable alternative compared to FLASH even in the 200μm URI regime on a clinical 3T MR system, provided that care is taken with the demands imposed by the bSSFP sequence.
Acknowledgements
This work was funded by the Swiss National Fund PP00P3_176984 and supported by the German Ministry of Education (BMBF; KKNMS German competence network for multiple sclerosis).References
1. Pfefferbaum A, Sullivan EV, Adalsteinsson E, et al. Postmortem MR imaging of formalin-fixed human brain. Neuroimage 2004;21(4):1585-95.
2. Miller KL, Stagg CJ, Douaud G, et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 2011;57(1):167-181.
3. Tendler BC, Hanayik T, Ansorge O, et al. The Digital Brain Bank, an open access platform for post-mortem datasets. bioRxiv preprint doi: https://doi.org/10.1101/2021.06.21.449154
4. Edlow B, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data 2019 Oct 30;6(1):244.
5. Weigel M, Dechent P, Galbusera R, et al. Imaging multiple sclerosis pathology at 160μm isotropic resolution by human whole-brain ex vivo magnetic resonance imaging at 3 T. Sci Rep 2021 Jul 29;11(1):15491. doi: 10.1038/s41598-021-94891-1.
6. Luciano NJ, Sati P, Nair G, et al. Utilizing 3D Printing Technology to Merge MRI with Histology: A Protocol for Brain Sectioning. J Vis Exp 2016 Dec 6;(118).
7. Absinta M, Nair G, Filippi M, et al. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J Neuropathol Exp Neurol. 2014;73(8):780-8.
8. Griffin AD, Turtzo C, Parikh G, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142(11):3550-3564.
9. Wang X, Roeloffs V, Merboldt KD, et al. Single-shot Multi-slice T1 Mapping at High Spatial Resolution – Inversion-Recovery FLASH with Radial Undersampling and Iterative Reconstruction. The Open Medical Imaging Journal 9(1):1-8
10. Scheffler K. On the transient phase of balanced SSFP sequences. Magn Reson Med 2003 Apr;49(4):781-3. doi: 10.1002/mrm.10421.
11. Bangerter NK, Hargreaves BA, Vasanawala SS, et al. Analysis of multiple-acquisition SSFP. Magn Reson Med. 2004 May;51(5):1038-47. doi: 10.1002/mrm.20052.
Figures
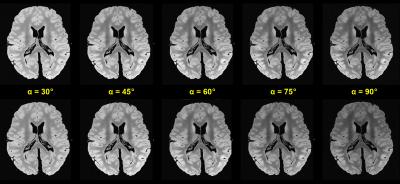
Upper row: With “optimal”, automatic window-levelling for each dataset. Lower row: Same acquisitions as above, however, with identical window-levelling, which aids in the visual comparison of achievable signal intensity.
The signal maximum is near 50°, however, a higher flip angle of 80° was chosen since it provides a higher T2 contrast contribution.
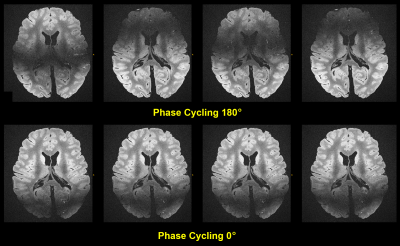
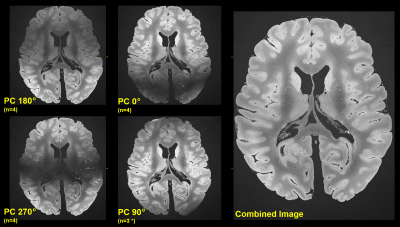
Figure 3: The smaller image patches on the left side present the mean images for a given phase cycling (PC) as noted (full 200μm resolution). Combining all images – here in the simplest way by taking the arithmetic mean (Ref. 11) – leads to an bSSFP image of homogenous signal intensity, despite of the ultra-high resolution acquired on a clinical system over 27.5h using a “large TR” of 13ms.
* Due to acquisition time constraints only 3 repetitions were measured with 90° phase cycling.