1904
Phantom Development for Comparison of Thermal Mapping1The xMR Labs, Physics and Astronomy, Western University, London, ON, Canada, 2Research and Development, Synaptive Medical, Toronto, ON, Canada, 3Medical BioPhysics, Western University, London, ON, Canada
Synopsis
Thermal Mapping using MRI is a non-invasive method for probing the body and has both diagnostic and interventional applications. If conducted on more accessible low field strength systems, these can improve the accessibility of the method for wider use. In order to validate and compare thermal mapping, a temperature control phantom was developed with tools for heating the phantom and externally tracking the temperature of the phantom. A heating study was performed with the phantom at two temperatures, and the thermometry methods showed good connection to the change in temperature in the phantom.
Introduction
The non-invasive and tissue dependent nature of Magnetic Resonance (MR) as an imaging modality provides several opportunities for unique applications [1]. MR provides stellar soft tissue contrast and can highlight tissue properties such as elasticity or permeability. Of particular interest is the sensitivity that various MR parameters have to tissue temperature, which allows for the generation of temperature maps that can be used for the monitoring of thermal therapies [2]. Generally, thermal mapping has been implemented using high main magnetic fields as to provide high Signal-to-Noise and overcome some of the challenges present at low field, although high quality low field imaging is an active area of study [3,4]. Should a method be developed (or current methods refined) which provide comparable image quality at low main magnetic field, there is potential for the proliferation in the use of thermal mapping for both diagnostic and interventional applications due to improved accessibility and the reduced cost associated with lower field strength systems. To validate and compare thermal mapping at a variety of field strengths, an MR safe phantom which can track temperature to cross reference with acquisitions from the scanner is required. In addition, the ability to control the temperature of the phantom is desirable, as this allows for the testing of thermometry during active heating. In this study, we develop a temperature control phantom that can be used to mimic certain tissue properties and can be used to validate and compare different thermal mapping methods.Methods
The body of the phantom was constructed of two nesting Rubbermaid Tupperware containers (1.5L and 0.750 L sizes respectively), with the buffer between the two providing the insulating effects. To hold the containers in place, a lid was designed in SolidWorks [Dassault Systemes] and 3D printed [Pro2, RAISE 3D] using ABS plastic, to which the two containers were epoxied to. The lid also includes a pair of probe insert locations where a fibre optic thermometer can be placed. The NOMAD-Touch [Qualitrol] system was used as the optical thermometer (±1 °C; ±0.1 °C Resolution, -20 to 50 °C range, calibrated using a monitored hot water bath). The last component on the lid is a holder for a 10 ohm through hole thin film resistor [Riedon] which can output 50W of power (-55 to 155 °C operating range, ±1% tolerance). Once attached to the lid, the resistor was held in place using a plastic screw and the phantom was filled with doped water (1mM MnCl2, 0.163mM CuSO4, 76.3mM NaCl, corresponding to T1 = 600ms, T2 = 150ms).Images were collected on a Cryogen-Free 0.5T head only MR scanner [Synaptive Medical Inc.] fitting the phantom and a reference vial inside a human sized head RF array. A Multiple Echo Gradient Echo pulse sequence (matrix size = 200x110, FOV=22cm, slice thickness=3mm, TR=27ms, TE at 7.0, 13.7, and 20.4ms) acquired images over a 21-minute period while 6-minute rounds of current (20V, 1.77A) were applied to the resistor- with a 10 second pull of the paddle mixing mechanism homogenized the temperature throughout- with 3 minute acquisitions between rounds of heating. A python pipeline was created to read in the files and match them to the temperature monitored from the NOMAD-Touch system.
Results
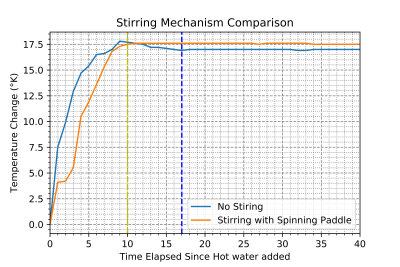
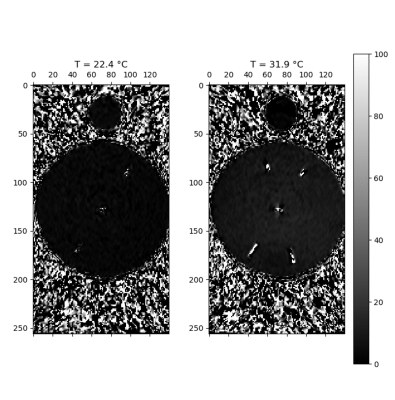
The temperature control phantom is shown in Figure 1, with the validation of the homogenization of temperature using the spinning paddle method displayed in Figure 2, guiding the operating protocol of using the stirring mechanism to homogenize the temperature inside the phantom. Figure 3 displays a structural MR scan of the phantom using a coronal T1 scan, where the resistor location along with the probe inserts and mixing mechanism can be seen. Further, Figure 4 shows the results of a heating test, where the acquisition from a thermometry pulse sequence is compared at two solution temperatures measured on the thermal.Discussion & Conclusion
This study has encompassed both the development and validation of a phantom which has properties favorable for producing thermal maps using MR. By validating the mechanism for homogenizing the temperature (Figure 2), a protocol for remotely mixing the solution so that the temperatures measured using thermometry pulse sequence was confirmed, ensures that the phantom can be set to any temperature, so long as the heating method is consistent. Use of a resistor for heating the phantom proved effective, however as seen in Figure 3, susceptibility artefacts created by the resistor warrant pause. As the resistors are metallic on the outside, they limit how close measurements can get, as these distortions affect the signal measured nearby. Although this remains MR safe, to mitigate artefacts, a high-power ceramic resistor could be used.Future improvements involve inclusion of additional probe locations to the lid of the phantom, and integration of permanent reference vials on the sides. This, along with variation of the viscosity of the solution in the phantom will offer more flexibility in controlling the favorable properties for thermometry depending on the specific method used.
Through a simple temperature dependence validation (Figure 4), the phantom adequately matched a Multi-Echo GRE thermometry sequence, and with optimizations to the acquisition could evaluate the accuracy to which the pulse sequence is able to measure the temperature.
Acknowledgements
The authors would like to acknowledge support form NSERC and the Ontario Research Fund.
References
[1] R. W. Brown, et al. “Magnetic resonance imaging: physical principles and sequence design.” John Wiley & Sons, 2014.
[2] V. Rieke and K. B. Pauly. "MR thermometry." Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 27.2 (2008): 376-390
[3] A. E. Campbell-Washburn, et al. “Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI.” Radiology, 293(2):384–393, 2019.
[4] Panther, A., et al. (2019). A Dedicated Head-Only MRI Scanner for Point-of-Care Imaging. ISMRM Proceedings.
Figures