1902
Evaluation of a Novel 8-Channel Receive Coil for Speech Production MRI at 0.55 Tesla.1Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 3Siemens Medical Solutions, Los Angeles, CA, United States, 4Stark Contrast, Erlangen, Germany
Synopsis
MRI has enabled significant advances in our understanding of human speech production. This application benefits from a lower magnetic field due to reduced off-resonance effects at air-tissue interfaces. Here, we evaluate the SNR and parallel imaging performance of a dedicated speech coil at 0.55 Tesla, and compare it to that of a head-neck coil. Over the upper airway regions of interest, the dedicated coil shows approximately 1.3- to 4.6-fold improvement in SNR efficiency compared to the head-neck coil.
Introduction
MRI is a powerful tool to visualize the complex coordination of articulators, enabling advances in speech science and clinical applications1. The linear scaling of susceptibility variation with field strength makes low field beneficial, especially because the features of interest are at the air-tissue interfaces2. Multi-channel local coils also provide increased SNR, and the ability to perform parallel imaging, increasing scan efficiency3.In this work, we evaluate a custom upper airway (UA) coil for 1H imaging at 0.55 Tesla (23.6 MHz) and evaluate its performance in comparison with a head/neck (HN) coil over nine upper airway volumes-of-interest (VOIs). We also evaluate the parallel imaging performance for the dedicated UA coil using Cartesian SENSE with uniform and variable density undersampling. Similar studies at 1.5 and 3 Tesla showed significant SNR improvement with localized speech coils (up to 5-fold) 4-5.
Methods
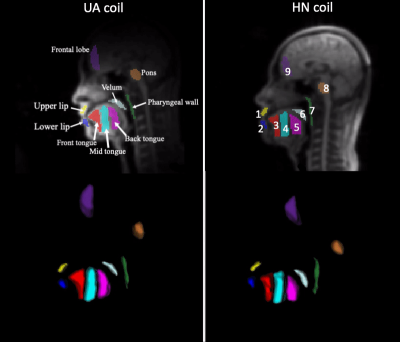
Experimental Methods: Experiments were performed using a whole body 0.55T system (prototype MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate). The dedicated 8 channel UA coil (Stark Contrast, Germany) and the standard HN coil with 16 channels are shown in Figure 1. 3D volumes of the upper airway were acquired using 3D gradient echo imaging. Imaging parameters were: flip angle = 10o, TE = 5 ms, TR = 10 ms, FOV = 32x32x16 cm3, resolution = 2.5 x 2.5 x 5 mm3, receiver bandwidth = 150 Hz/Px, and ky and kz phase encoding along A-P and R-L directions, respectively. Four subjects were scanned in a supine position under a protocol approved by our Institutional Review Board, after providing written informed consent.Data Analysis: Pre-scan noise was acquired with 256*128 samples per channel and used for pre-whitening. SNR maps were computed with the following equation6:
$$ SNR =\frac{ \sqrt{2} \left| \bf{b}^{H}\bf{p} \right|} {\sqrt{\left( \bf{b}^{H} \bf{b} \right) } } $$
where p is the vector of complex image values for each coil, b is the vector of complex coil sensitivities. Image and coil sensitivity data were pre-whitened prior to SNR evaluation. To compare the SNR efficiency of both coils, each coil was first compared to the integrated body coil by performing the imaging sequence without patient repositioning. This allowed calculation of SNR gains for each of UV and HN coils relative to the body coil. Subsequently, the ratio of SNR gains for each coil was obtained.
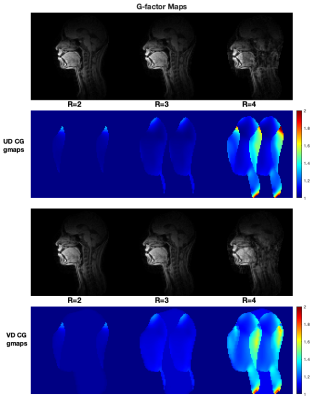
Figure 2 illustrates the 9 VOIs used for SNR evaluation. Each VOI was edited using the 3D segmentation tools of ITK-SNAP7. For parallel imaging evaluation, g-factors were calculated for conjugate gradient (CG) SENSE with uniform density undersampling (UD), and for variable density (VD) Cartesian trajectory using 32 central lines. We tested acceleration factors of 2, 3, and 4. For CG SENSE, the g-factor at pixel ρ is defined as8:
$$ g_{\rho} = \sqrt{(E^{H}E)^{-1}_{\rho,\rho} \: (E^{H}E)_{\rho,\rho}} $$
where E is the encoding matrix as defined in [9].
Results
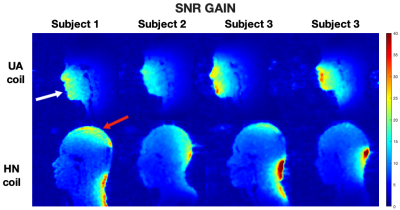
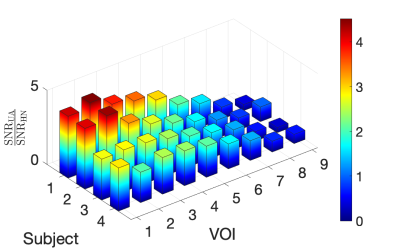
Figure 3 compares relative SNR for the UA and HN coil in all four subjects. The UA coil provides higher SNR gain in the upper airway regions whereas the HN coil provides higher SNR gain in the brain regions, as expected. This is supported by the quantitative measure of SNR gain as shown in Figure 4. The largest gains were in the upper and lower lip areas. The HN coil outperforms the UA coil in regions closer to the brain, such as the frontal lobe.Figure 5 contains g-factor maps, along with reconstructed images. For R = 2 and 3, both UD and VD trajectories have g-factor below 1.1 in the upper airway region. For R = 4, the g-factor are higher for both sampling schemes.
Discussion
The placement of coil elements allows for a high SNR gain near the vocal tract articulators, while the gain is negligible near the pharyngeal wall. The high SNR observed near the articulators is beneficial to upper airway imaging, as it mitigates g-factor noise amplification allowing for better image quality10.Increasing SNR by minimizing coil size is limited by coil loses. In the present coil element size is optimized for deeper structures. The quality factor Q0 is optimized and the highest loading is achieved by anatomical shaping to keep distance to the skin small. For even faster imaging, a design with more elements can help acceleration (if g-factors allow). This could give higher SNR near the surface. With a tradeoff for loading factor at this low frequency, coil losses contributions will increase significantly and SNR at depth will decrease. Inductive coupling between elements at low frequency will also increase noise: this effect can be worse for more elements.
Conclusion
The novel UA coil showed a 1.3-4.6-fold improvement in SNR over the HN coil in VOIs relevant to speech production. The g-values for the UA coil in the upper airway regions are below 1.1 for R = 2 and 3, for uniform and variable density CG SENSE. This is favorable for high-performance speech production dynamic MRI at 0.55 Tesla.Acknowledgements
We acknowledge grant support from the National Science Foundation (#1828736) and research support from Siemens HealthineersReferences
1. Lingala SG, Sutton BP, Miquel ME, Nayak KS. Recommendations for real-time speech MRI. Journal of Magnetic Resonance Imaging. 2016;43(1):28–44. doi:10.1002/jmri.24997
2. Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.0T. Journal of Magnetic Resonance Imaging. 2006;24(4):735–746. doi:10.1002/jmri.20698
3. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magnetic Resonance in Medicine. 1990;16(2):192–225. doi:https://doi.org/10.1002/mrm.1910160203
4. Kim Y-C, Hayes CE, Narayanan SS, Nayak KS. Novel 16-channel receive coil array for accelerated upper airway MRI at 3 Tesla. Magnetic resonance in medicine. 2011;65(6). doi:10.1002/mrm.22742
5. Lingala SG, Zhu Y, Kim Y, Toutios A, Narayanan S, Nayak KS. A fast and flexible MRI system for the study of dynamic vocal tract shaping. Magnetic Resonance in Medicine. 2017;77(1). doi:10.1002/mrm.26090
6. Kellman P, McVeigh ER. Image reconstruction in SNR units: A general method for SNR measurement. Magnetic Resonance in Medicine. 2005;54(6):1439–1447. doi:10.1002/mrm.20713
7. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. doi:10.1016/j.neuroimage.2006.01.015
8. Sheng J, Ying L, Liu B, Abdelsalam E, Sheng J, Ying L. G-factor Maps of Conjugate Gradient SENSE Reconstruction. 2008.
9. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magnetic Resonance in Medicine. 2001;46(4):638-651. doi:10.1002/mrm.1241
10. De Zwart JA, Ledden PJ, Van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-Noise Ratio and Parallel Imaging Performance of a 16-Channel Receive-only Brain Coil Array at 3.0 Tesla. Magnetic Resonance in Medicine. 2004;51(1):22–26. doi:10.1002/mrm.10678
Figures