1894
Parahydrogen-Based Hyperpolarization of a Pyruvate Ester to 25% Within an MRI System Using SAMBADENA1Radiology - Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 2German Cancer Consortium (DKTK) partner site Freiburg, German Cancer Center (DKFZ), Heidelberg, Germany, 3University Medical Center Freiburg, Freiburg, Germany, 43Section Biomedical Imaging, Molecular Imaging North Competence Center (MOINCC), Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein and Kiel University, Kiel, Germany, 5Max Planck Institute for Biophysical Chemistry, Göttingen, Germany, 6Center for Biostructural Imaging of Neurodegeneration, University Medical Center Göttingen, Göttingen, Germany
Synopsis
Parahydrogen (pH2) and Synthesis Amid the Magnet Bore Allow Dramatically Enhanced Nuclear Alignment (SAMBADENA) and have recently enabled hyperpolarization (HP) of 13C and subsequent in vivo administration of the produced contrast agent inside an MRI system. pH2 Induced Polarization (PHIP) by Side-Arm Hydrogenation (PHIP-SAH) has extended the portfolio of PHIP agents to metabolically-active molecules such as acetate, lactate, and pyruvate, but typically requires hydrogenation in organic solvents like chloroform. Here we present our new, solvent-compatible SAMBADENA setup and preliminary results of the 13C HP in chloroform of the PHIP-SAH molecule cinnamyl-pyruvate – opening the pathway towards metabolic studies with SAMBADENA.
Introduction
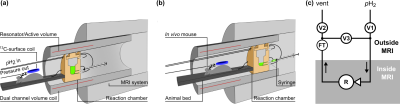
MRI is a powerful technique, but sensitivity limits its use to imaging mostly 1H in water and lipids. Hyperpolarization (HP) allows 104-105-fold signal enhancements at clinical magnetic fields, which has enabled entirely new applications such as real-time metabolic MRI using carbon‑13. Parahydrogen (pH2) Induced Polarization (PHIP) is promising, because of low-cost and fast production and recently, PHIP by Side-Arm Hydrogenation (-SAH) enabled HP of pyruvate but typically requires hydrogenation in organic solvents (e.g., chloroform).1 Synthesis Amid the Magnet Bore Allows a Dramatically Enhanced Nuclear Alignment (SAMBADENA) and has enabled HP of 13C and subsequent in vivo administration of an agent inside an MRI system (Fig. 1a,b).2,3 Here we present our new, solvent compatible setup and preliminary results of the 13C HP in chloroform of the PHIP-SAH molecule cinnamyl-pyruvate4 – a precursor for metabolic imaging with HP pyruvate.METHODS
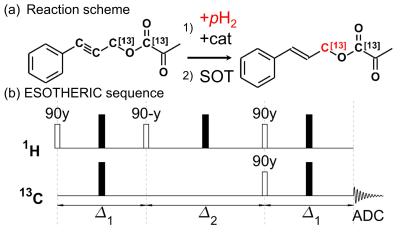
Setup: The SAMBADENA setup has been described in detail before (Fig. 1).2,3 A preclinical 7T MRI (ParaVision 6.1, BioSpec 70/20, Bruker) was used with a 1H-13C transmit-receive volume rat coil (7.2cm x 10cm (ID x length), Rapid Biomed). HP (hydrogenation and spin-order transfer (SOT)) was hosted in a custom-built high-pressure reactor mounted in the isocenter of the MRI magnet. In contrast to previous reports, the reactor was made from polyether ether ketone (PEEK) instead of polysulfone (PSU) and other setup components were replaced by chloroform-resistant materials, too. Tubing and fittings were of PTFE and PEEK or ETFE, respectively. All sealing materials were exchanged by the perfluoro elastomer FFKM. To protect the magnetic valves, a stainless-steel fluid trap was designed and installed in the outlet of the reactor, outside of the magnet.Experiments: pH2 was enriched to »90% at ~21 K as described previously.5 Samples were prepared in chloroform with 2 mM of rhodium-based catalyst [Rh(dppb)(COD)]BF4, CAS: 79255-71-3) and 2 mM substrate precursor (3-Phenyl-2-[1-13C]propyn-1-yl [1-13C]pyruvate) that yielded [1-13C]cinnamyl [1‑13C]pyruvate (CPYR) upon pH2 addition (Fig. 2).4 Hydrogenation with pH2 took place at 7 T, room temperature, and ≈15 bar. To this end pH2 was injected into the reactor and passed the solution for 2 s until the pressure was equilibrated and the gas flow stopped (valve V1 open, Fig. 1b). The start of SOT was delayed by a variable time (0 – 50 s; valves V1 + V3 open) such that the total hydrogenation time, tHydr, including the 2 s pH2 injection, was varied between 2 s and 52 s. At the end of tHydr, the ESOTHERIC sequence was employed to transfer pH2-nascent I1zI2z spin order to the [1-13C]-cinnamyl site (Fig. 2). Note that the relayed 13C-13C transfer to polarize the [1‑13C]pyruvate site as suggested by Korchak, Glöggler and coworkers was not implemented yet.4
Quantification: HP was quantified by comparing the 13C signals from hyper- and thermally-polarized samples. The reaction kinetics and HP as function of tHydr were further investigated by fitting a model to the data:2
$$P_{13C}\left(t_{Hydr}\right)={P_{max}}\cdot\left(\left(T_{Hydr}/T_{relax}\right)-1\right)^{-1}\cdot\left[exp\left(-\left(t_{Hydr}-t_{0}\right)/T_{Hydr}\right)-exp\left(-\left(t_{Hydr}-t_{0}\right)/T_{relax}\right)\right]$$
Pmax is the maximum polarization value reached if no relaxation were present. t0 is a time offset (delivery and dissolution of pH2, build-up of pressure, and activation of catalyst). THydr and Trelax are time constants of the hydrogenation and relaxation of the 1H spin order, respectively.
For calibrations of the MRI setup and quantification of HP, a reference 13C solution held in a 1.5 mL Eppendorf vial placed inside the reactor was used (4 M [1-13C]sodium acetate in 1.5 mL H2O, 99 % 13C, CAS: 23424-28-4, Gd-doped, T1 = 832 ms).
Results
The setup resisted the chloroform solutions used in the experiments well and no adverse effects were visible. The maximum 13C polarization observed for the methylene site of CPYR was P13C = (25 ± 2.7) %. The fitted kinetics model suggested the following parameters: Pmax = (42.3 ± 4.7) %, THydr = (3.8 ± 0.7) s, Trelax = (12.3 ± 2.0) s, and t0 = (3.8 ± 0.1) s. Hence, at the maximum of the curve (at tHydr = 10.1 s), (81.6 ± 9.0) % of the precursor was reacted. With a faster (instantaneous) and complete hydrogenation P13C = Pmax = (42.3 ± 4.7) % could be achieved. A transfer to the [1-13C]pyruvate could not be achieved yet, because the RF power of the MRI was too low to excite both 13C sites with the 13C pulses.6Discussion
Although reactions took place at 23 °C, remarkably high polarizations were observed that can most likely be improved further at elevated reaction temperatures.2 The relayed 13C-13C SOT from the side arm to the pyruvate could likely be conducted with (frequency) modulated pulse schemes or by playing out 13C pulses resonating at the two different frequencies subsequently. Ultimately, for biomedical application cleavage of the side arm and a rapid purification to extract a clean solution of pyruvate (or other agents) also need to be implemented in future works.1,7Conclusion
In conclusion, SAMBADENA of PHIP-SAH molecules was enabled and can likely be improved further along with reaction temperature. To yield clean solutions of hyperpolarized agents, additional work is needed – and currently ongoing in our lab. However, solving these mostly technical issues is very promising and may transform in situ HP with SAMBADENA into a valuable method for metabolic MRI.Acknowledgements
This work received funding support from the Research Commission of the University Medical Center Freiburg (SCHM2146-20), the German Cancer Consortium (DKTK) and the German Research Foundation (DFG grant numbers: SCHM 3694/1-1, HO-4602/2-2, HO-4602/3, SFB1479).
References
(1) Reineri, F.; Boi, T.; Aime, S. ParaHydrogen Induced Polarization of 13C Carboxylate Resonance in Acetate and Pyruvate. Nat. Commun. 2015, 6, 5858. https://doi.org/10.1038/ncomms6858.
(2) Schmidt, A. B.; Berner, S.; Schimpf, W.; Müller, C.; Lickert, T.; Schwaderlapp, N.; Knecht, S.; Skinner, J. G.; Dost, A.; Rovedo, P.; Hennig, J.; Elverfeldt, D. von; Hövener, J.-B. Liquid-State Carbon-13 Hyperpolarization Generated in an MRI System for Fast Imaging. Nat. Commun. 2017, 8, 14535. https://doi.org/10.1038/ncomms14535.
(3) Schmidt, A. B.; Berner, S.; Braig, M.; Zimmermann, M.; Hennig, J.; Elverfeldt, D. von; Hövener, J.-B. In Vivo 13C-MRI Using SAMBADENA. PLOS ONE 2018, 13 (7), e0200141. https://doi.org/10.1371/journal.pone.0200141.
(4) Korchak, S.; Yang, S.; Mamone, S.; Glöggler, S. Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by Utilizing Para-Hydrogen. ChemistryOpen 2018, 7 (5), 344–348. https://doi.org/10.1002/open.201800024.
(5) Hoevener, J.-B.; Bär, S.; Leupold, J.; Jenne, K.; Leibfritz, D.; Hennig, J.; Duckett, S. B.; von Elverfeldt, D. A Continuous-Flow, High-Throughput, High-Pressure Parahydrogen Converter for Hyperpolarization in a Clinical Setting. NMR Biomed. 2013, 26, 124–131.
(6) de Maissin, H.; Berner, S.; Ivantaev, V.; Hoevener, J.-B.; Hennig, J.; von Elverfeldt, D.; Schmidt, A. B. Dramatic Effect of Pulse Length and Bandwidth on the Efficiency of Pulsed Spin-Order-Transfer Sequences at High Field. In Proc. Intl. Soc. Mag. Reson. Med. 29; Online, 2021; p 3805.
(7) Knecht, S.; Blanchard, J. W.; Barskiy, D.; Cavallari, E.; Dagys, L.; Dyke, E. V.; Tsukanov, M.; Bliemel, B.; Münnemann, K.; Aime, S.; Reineri, F.; Levitt, M. H.; Buntkowsky, G.; Pines, A.; Blümler, P.; Budker, D.; Eills, J. Rapid Hyperpolarization and Purification of the Metabolite Fumarate in Aqueous Solution. Proc. Natl. Acad. Sci. 2021, 118 (13). https://doi.org/10.1073/pnas.2025383118.
Figures