1892
Investigating metabolite regional dependencies in the frontal lobe: overlapping small and large voxels1Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 2Department of Psychiatry, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 4Mathison Centre for Mental Health Research and Education, University of Calgary, Calgary, AB, Canada, 5Department of Radiology, University of Calgary, Calgary, AB, Canada, 6Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada, 7Provincial Addiction and Mental Health (PAMH) Portfolio, University of Calgary, Calgary, AB, Canada
Synopsis
Small anatomically specific voxels in cerebral cortex require a large number of averages to quantify metabolites by magnetic resonance spectroscopy. It is unclear whether cortical regional variability in metabolite concentration requires small voxels, or whether a larger voxel which incorporates the region of interest can provide adequately representative measures with fewer averages. Concentrations of glutamate, glx, creatine, choline, myo-inositol and N-acetyl-aspartate (NAA) were correlated within subjects between an overlapping small (15x15x15mm) and large (30x30x30mm) voxel. There was a significant correlation between creatine measures, while choline showed a positive trend. There was no correlation between glutamate, glx, NAA, and myo-inositol.
Introduction
Magnetic Resonance Spectroscopy (MRS) experiments are often interested in quantifying metabolites in a small anatomical region of interest (ROI). When investigating cortical regions, where boundaries and physiological differences are not clear, such as the dorsolateral prefrontal cortex (DLPFC), the local anatomical specificity of a MRS voxel is challenging in terms of accurate and reliable voxel placement. Evoking the question: is a small voxel necessary, or can a larger voxel be representative?This question has important practical implications in terms of data quality and signal-to-noise (SNR). As SNR is proportional to the volume of the voxel and the square root of the number of averages ($$$SNR \propto B^{0}V\sqrt{N}$$$)1, it is more efficient to increase voxel size rather than increase scan duration, which comes with additional risk of motion. It is unclear whether this compromises the regional sensitivity of the measure, due to regional variability in cortical metabolite concentrations and differences tissue composition.
This study investigated the correspondence of metabolite levels between the two overlapping voxels: a larger voxel with higher SNR and shorter scan time and a smaller voxel placed within it with lower SNR despite considerably longer scan time.
Methods
Acquisition
A T1-weighted anatomical image was acquired for voxel placement and voxel segmentationTwo short-echo Point REsolved SpectroScopy (PRESS) acquisitions were acquired in the right DLPFC from 19 participants. For both scans, the parameters were: TR=1800ms, TE=35ms, 4096 points, 5kHz bandwidth. The small voxel was 15x15x15mm3 with 200 averages. The large voxel was 30x30x30mm3, with 64 averages. An illustrative voxel placement is shown in Figure 1A.
Anatomical Registration
The voxels were coregistered to the T1 and segmented using the Gannet CoRegStanAlone function2 and SPM123.MRS Processing
PRESS data were pre-processed using FID-A4, metabolites were quantified using LCModel5, tissue corrected and CSF-corrected for molar concentrations6,7. The metabolites of interest in this study were: glutamate, choline, creatine, myo-inositol, N-acetyl aspartate (NAA) and glutamate-glutamine (glx). Example spectra are shown in Figure 1B.The SNR of the large voxel was substantially higher than the SNR of the small voxel. To investigate the impact of SNR on metabolite level correspondence, a secondary analysis matched the SNR between the large and small voxels by only analysing, the first 8 averages from the large voxel data which were then pre-processed and quantified using the same routine.
Analysis
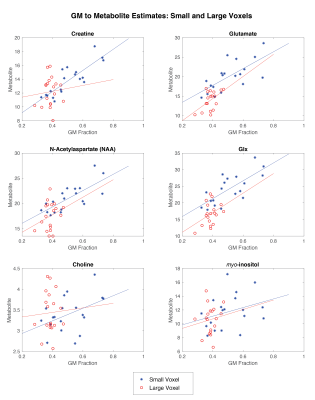
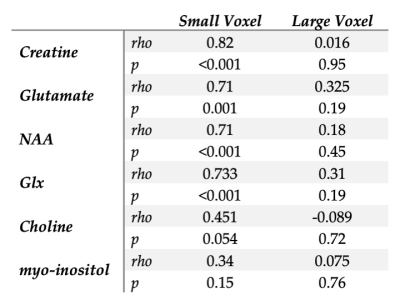
Metabolite correspondence was assessed using Spearman’s rho, comparing the CSF-corrected metabolites in the small voxel to that of the large voxel, with both the full SNR large voxel and the matched SNR large voxel.To investigate the reliance of metabolite estimates on tissue composition, Spearman’s rho correlations were run between grey matter (GM) fraction (as a proportion of total tissue) and metabolite estimates. These were run separately for the small voxel and large voxel (with all averages).
Results
Metabolite correspondence
Creatine was the only metabolite significantly correlated between the two voxels (rho=0.53, p=0.021) though Cho was trend level (Figure 2).Repeating these correlations with the reduced SNR large voxel, the correlation between myo-inositol in the large voxel and small voxel became significant (rho=0.29, p=0.014) and creatine remained significant (rho=0.48, p=0.037). The correlations for creatine, NAA, glx and choline are highly consistent between the SNR degraded voxel and the full SNR voxel. These are illustrated in Figure 3.
Tissue contribution
There was proportionally more GM in the small voxel than the large voxel, (t=4.087, p<0.0001).The correlations between GM fraction and metabolite estimates are shown in Figure 4 and reported in Table 1.
Discussion
These findings evidence high local regional variability in metabolites in the frontal cortex. Given the small voxel was encompassed by the larger voxel, it is perhaps surprising there was not a higher correspondence between the metabolite concentrations between the voxels. Differences in SNR and tissue composition appear to contribute to this lack of correspondence, and it is worth noting that the small voxel is ~12% the volume of the large voxel.Because even with only 64 averages the large voxel has substantially higher SNR than the small voxle (average SNRLarge=33.53, SNRSmall=14.11) the analysis was repeated with more closely matched SNRs by only including 8 averages of the large voxel (average SNRReduced=14.95). In this analysis, a correlation for myo-inositol appeared and the creatine correlation remained. Similar effects held for NAA, choline and Glx.
Differences in tissue composition between the two voxels likely impacts the results given there was a positive correlation between GM fraction and metabolite estimates for all metabolites in the small voxel (though not significant for myo-inositol or choline). It is likely the lack of correlations in the large voxel are a function of the lack of variance in GM content. Given the correlations between GM fraction and metabolite estimates, either co-varying for tissue content or developing tissue correction factors for metabolites2 are approaches to account for voxel-tissue composition. Accounting for difference in metabolite concentration between GM and WM will assist in using representative larger voxels but it is unclear if this is sufficient to fully account for this effect.
This study evidences marked variability of metabolite estimates within the DLPFC. This variability may be somewhat accounted for by tissue composition differences, a factor worthy of further investigation.
Acknowledgements
We wish to acknowledge a Hotchkiss Brain Institute International Recruitment Fellowship and Cumming School of Medicine Postdoctoral Fellowship to Marilena M. DeMayo.This project was funded by the Department of National Defence.References
1 Kreis, R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed 17, 361-381, doi:10.1002/nbm.891 (2004).
2 Harris, A. D., Puts, N. A. & Edden, R. A. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging 42, 1431-1440, doi:10.1002/jmri.24903 (2015).
3 Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839-851, doi:10.1016/j.neuroimage.2005.02.018 (2005).
4 Simpson, R., Devenyi, G. A., Jezzard, P., Hennessy, T. J. & Near, J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med 77, 23-33, doi:10.1002/mrm.26091 (2017).
5 Provencher, S. W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14, 260-264, doi:10.1002/nbm.698 (2001).
6 Near, J. et al. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed 34, e4257, doi:10.1002/nbm.4257 (2021).
7 Gasparovic, C. et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55, 1219-1226, doi:10.1002/mrm.20901 (2006).
Figures
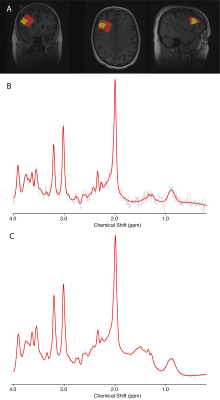
Figure 1.
A) An example voxel placement
B) Exemplar spectrum from the small voxel
C) Exemplar spectrum from the large voxel
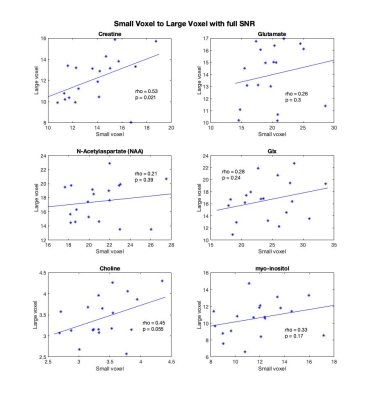
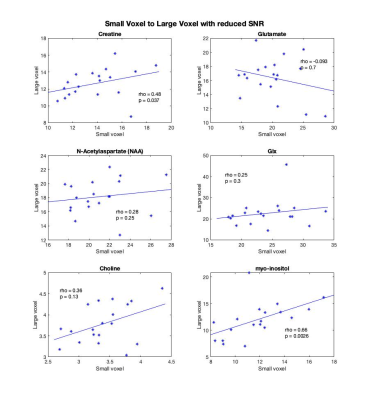
Figure 3. Spearman's rho correlations between the metabolite estimates within the degraded SNR large voxel, 8 averages (y-axis), and small voxel (x-axis). Creatine remained significantly correlated and myo-inositol became significantly correlated between the two voxels.