1886
Advancing SABRE Toward In Vivo Imaging: Phantom Chemical Conversion of Hyperpolarized [1-13C]-Pyruvate1Chemistry, North Carolina State University, Raleigh, NC, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Vizma Life Sciences, Chapel Hill, NC, United States, 4Chemistry, Physics, Biomedical Engineering, North Carolina State University, Raleigh, NC, United States
Synopsis
This work illustrates current advancements in SABRE hyperpolarized pyruvate toward in vivo imaging, demonstrating that significant levels of polarization achieved on pyruvate enable dynamic spectroscopy and imaging of downstream chemical products. Specifically in this study we show the monitoring of chemical conversion of pyruvate to pyruvate hydrate using a pH change and pyruvate to CO2 by reaction with H2O2. While the current work only focuses on the study of the chemical conversion of hyperpolarized substrate in a phantom, these studies lay the groundwork for future in vivo injections that mirror these chemical conversion processes.
INTRODUCTION
The emerging technology of hyperpolarized (HP) MRI has enabled broad advances in the field of metabolic imaging, generating contrast agents that can be used to directly image metabolism in vivo to elucidate the metabolic distortion of cancer and other diseases1. HP [1-13C]pyruvate is the leading agent under investigation in a number of ongoing clinical trials, spearheaded by the advancements of dissolution Dynamic Nuclear Polarization (d-DNP)2, due to central nature of pyruvate metabolism in various diseased tissues3. However, HP-MRI has remained limited in scope due to the substantial cost (≈$2.5M) and long hyperpolarization production times (≈30 min) of d-DNP. The more recent emergence of parahydrogen (p-H2) based hyperpolarization and specifically Signal Amplification by Reversible Exchange address the inherent technical limitations of d-DNP by generating direct polarization on target substrates in room temperature solutions. This chemistry relies on reversible interactions between the target substrate, parahydrogen, and a polarization transfer catalyst to generate hyperpolarized signal. Groundbreaking work from Duckett et al. showed this chemistry can be applied to direct hyperpolarization of pyruvate4,5 and other 13C carboxylate molecules6, yielding up to 1.7% polarization on pyruvate. Building on this work we developed new methods to reach to >10% polarization on [1-13C]pyruvate using time-dependent temperature gradients7. In this work, we demonstrate how the polarization aided by these temperature gradients enables 13C spectroscopy and imaging of the chemical conversion of [1-13C]pyruvate to downstream products.METHODS
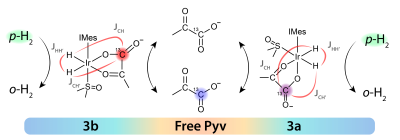
[1-13C]pyruvate is hyperpolarized by first pre-cooling the sample to boost initial polarization of pyruvate in the bound complex and then allowing the sample to warm during p-H2 bubbling to generate high polarization on free [1-13C]pyruvate in solution (Figure 1). Hyperpolarization transfer from the p-H2 derived hydrides to the target 13C spin is carried out at sub-μT magnetic fields using the method of SABRE in Shield Enables Alignment Transfer to Heteronuclei (SABRE-SHEATH) to reach the correct matching conditions for spin transfer. The sample is then either directly inserted into the magnet for detection or ejected into a secondary container for chemical transformation. In this study, we used NaBH4 as a reducing agent to convert pyruvate to lactate and H2O2 to observe conversion to ethanoic acid, CO2 and acetate. Studies were conducted in both a Bruker 4.7 T preclinical magnet at MGH and a variable field SSI magnet operating at 1.5 T.RESULTS AND DISCUSSION
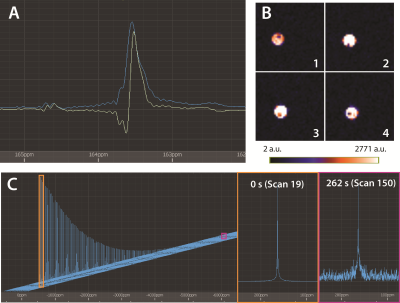
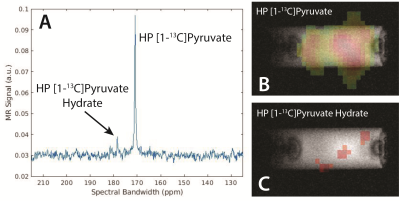
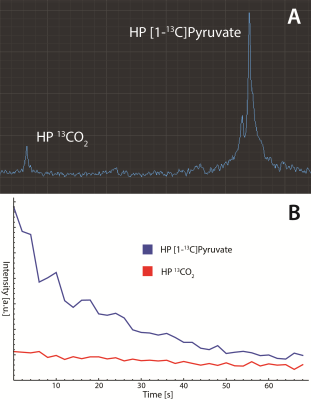
Figure 2a shows a hyperpolarized spectrum of [1-13C]pyruvate, along with benchmark images (Figure 2b) and dynamic acquisition (Figure 2c) of the hyperpolarized pyruvate signal. Using a 13C reference signal from concentrated [1-13C] ethyl acetate, the polarization of the hyperpolarized pyruvate inserted into the magnet is calculated as 5.8%. We use this signal to acquire multi-slice imaging data using a fast spin echo sequence, demonstrating enough signal and resolution to reveal the structure of the 0.72 mm bubbling capillary that winds around the NMR tube. Additionally, the dynamic acquisition shows that the generated signal lasts for over 4 minutes at 1.5 T, acquiring with 10° flip angles. In the 4.7 T magnet the hyperpolarized sample is ejected into a syringe containing a mixture of sodium borohydride and sodium borate in methanol, giving a change in pH of the sample that induces conversion of the hyperpolarized [1-13C]pyruvate signal into hyperpolarized pyruvate hydrate (Figure 3a). In Figure 3b and 3c this conversion is imaged using a CSI sequence, showing small amounts of conversion of the pyruvate to pyruvate hydrate with random distribution due to inefficient mixing of the syringe before insertion into the detection field. Figure 4 demonstrate spectroscopic monitoring of the conversion of hyperpolarized pyruvate to CO2, showing dynamic monitoring of the two peaks exhibiting more rapid decay of the pyruvate than CO2 signal due to the constant conversion of the pyruvate in solution during the experiment.CONCLUSION
The results shown in this work lay the groundwork for future translation of [1-13C]pyruvate hyperpolarized with SABRE-SHEATH into in vivo studies by demonstrating the successful conversion and detection of downstream chemical products. Specifically shown is the detection of chemical conversion of pyruvate to CO2 and pyruvate to pyruvate hydrate, including CSI imaging. After successfully establishing a threshold for SABRE polarization that would enable detection of metabolic conversion, future work will focus on the remaining translational hurdles necessary to bring SABRE-HP [1-13C]pyruvate into aqueous solutions and removal the iridium catalyst to generate biocompatible injectables for in vivo studies.Acknowledgements
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers NIH R21EB025313 and NIH R01EB029829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, we acknowledge funding from the Mallinckrodt Foundation, the National Science Foundation under award NSF CHE-1904780, and from the National Cancer Institute under award number NCI 1R21CA220137, as well as funding from the North Carolina Biotechnology Center in the form of a Translational Research Grant.References
(1) Vaeggemose, M.; Schulte, R. F.; Laustsen, C. Comprehensive Literature Review of Hyperpolarized Carbon-13 Mri: The Road to Clinical Application. Metabolites 2021, 11 (4).
(2) Ardenkjaer-Larsen, J. H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M. H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. 2003, 100 (18), 10158–10163.
(3) Serrao, E. M.; Brindle, K. M. Potential Clinical Roles for Metabolic Imaging with Hyperpolarized [1-13C]Pyruvate. Front. Oncol. 2016, 6 (MAR), 1–6.
(4) Iali, W.; Roy, S. S.; Tickner, B. J.; Ahwal, F.; Kennerley, A. J.; Duckett, S. B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chemie 2019, 131 (30), 10377–10381.
(5) Tickner, B. J.; Semenova, O.; Iali, W.; Rayner, P. J.; Whitwood, A. C.; Duckett, S. B. Optimisation of Pyruvate Hyperpolarisation Using SABRE by Tuning the Active Magnetisation Transfer Catalyst. Catal. Sci. Technol. 2020, 10 (5), 1343–1355.
(6) Tickner, B. J.; Ahwal, F.; Whitwood, A. C.; Duckett, S. B. Reversible Hyperpolarization of Ketoisocaproate Using Sulfoxide-Containing Polarization Transfer Catalysts. ChemPhysChem 2021, 22 (1), 13–17.
(7) Tomhon, P.; Abdulmojeed, M.; Adelabu, I.; Nantogma, S.; Kabir, M. S. H.; Lehmkuhl, S.; Chekmenev, E. Y.; Theis, T. Temperature Cycling Enables Efficient 13C SABRE-SHEATH Hyperpolarization and Imaging of [1-13C]Pyruvate. ChemRXiv 2021.
Figures