1884
Flow Effects on Spatial Spectral Excitation of Hyperpolarized Agents1Imaging Physics, MD Anderson Cancer Center, Houston, TX, United States, 2Neuroradiology Department, MD Anderson Cancer Center, Houston, TX, United States, 3Head & Neck Surgery, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Simulations were used to evaluate the effect of rapid vascular flow on spatial spectral pulses used for hyperpolarized MRI. Simulation results show that flow effects reduced the on-resonance excitation angle while increasing the off-resonance excitation in a velocity dependent manner. These excitation impacts will be particularly important when trying to estimate an arterial input function. Flow may also produce measurable lactate signal in flowing pyruvate, an effect that was observed in a hyperpolarized study of the thyroid.
Introduction
Hyperpolarized MRI provides a high signal injectable agent that is currently being investigated for its ability to image tissue metabolism(1). Spectral imaging is necessary to elucidate the chemical fate of the injected hyperpolarized compound. A common technique is to excite the desired metabolites separately through spectrally and spatially specific excitation pulses(2,3). Spectral spatial pulses can be long, typically around 10 msec, which could leave them susceptible to flow effects during excitation(4). Recent hyperpolarized studies of thyroid tumors(5) necessitated imaging the subclavian vein and carotid arteries which contained high-velocity vascular flow. This work seeks to use simulation to determine the impacts of high vascular flow on spatial-spectral pulses.Methods
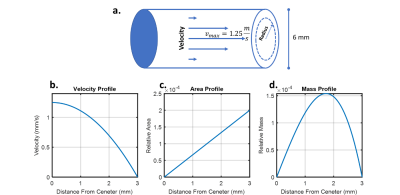
The Bloch equations were solved for a line of spins flowing along the slice direction for a range of velocities (2 to -2 m/s) for both on-resonance and off-resonance chemical shifts. The separation between on and off-resonance was set to match the pyruvate to lactate chemical shift difference at 3T. To account for laminar flow in the vessel, a Monte Carlo approach was used, pulling from the expected distribution of velocities weighted by their relative mass distribution. The vessel was modeled asa 6 mm diameter(6) vessel with a maximum velocity of 1.25 m/s(7), Figure 1. The resulting transverse magnetization from this ensemble of spins was then converted into an effective flip angle to estimate the apparent excitation angle.Results were validated in a patient scan using a Helmholtz-style 13C volume transmit “clamshell” coil (GE Healthcare) and an 8-channel paddle array(3). Hyperpolarized pyruvate was produced in a GE SPIN lab polarizer. Hyperpolarized images of pyruvate and lactate were acquired using multislice spectral-spatial excitation and echoplanar readout. Images had a 1.5 cm in-plane resolution, 8 mm slice thickness, 3s temporal resolution, and excitation angles ΘPyr = 20°; ΘLac= 30°(2). The patient was a 66-year-old male with biopsy-proven anaplastic thyroid carcinoma of the left lobe of the thyroid(5).
Results
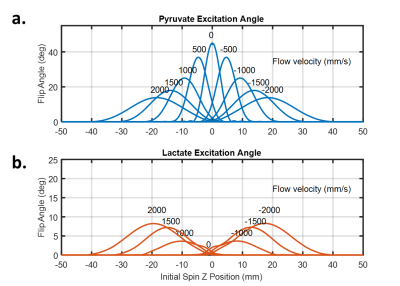
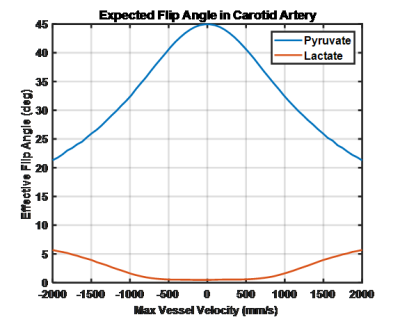
Slice profile simulations show the impact of flow on both the on-resonance and off-resonance metabolite, given a 45° excitation pulse, Figure 2. When the spin system is stationary the expected slice profile is observed with a maximal excitation angle of 45°. As flow speed increases, the maximal excitation angle is reduced and as shifts downstream of the direction of flow. A greater than 50% reduction in excitation angle at the maximal velocity explored (2 m/s) is observed. When compared to the on-resonance excitation, the off-resonance excitation angle increases with flow speed with an excitation angle nearly 1/5th of the on-resonance excitation at 2 m/s.In order to account for the range of expected velocities from laminar flow an effective excitation angle for the whole vessel was calculated based on the maximal vessel velocity as shown in Figure 3. Off and on-resonance excitation impacts due to flow effects are similar to Figure 2, but the excitation deviations are more modest. Notably at the maximum expected velocity in a healthy carotid artery is 1.25 m/s and as a result, the on-resonance excitation should be roughly 60% of the prescribed excitation angle while the off-resonance excitation should have risen to about 5%.
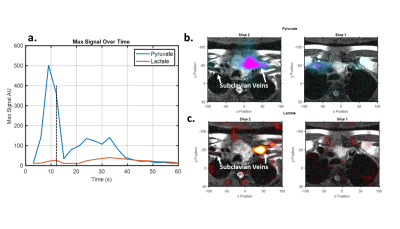
While having a small amount of the flowing lactate signal show up in the pyruvate excitation is unlikely to be an issue as there is not expected to be significant polarized lactate in the arterial supply the reduction in pyruvate excitation angle could impact arterial input function estimation. Having a small fraction of the vascular pyruvate show up in the lactate excitation could result in detected lactate signal. Indeed, such off-resonance excitation likely explains the early lactate signal observed in a hyperpolarized study of a thyroid cancer patient as seen in Figure 4. Early lactate signal is observed corresponding to the initial pyruvate bolus at 6, 9, and 12 seconds. Both pyruvate and lactate signals are well localized to the subclavian vein as it enters the superior vena cava. Additionally, the lactate and pyruvate signals spatially overlap suggesting that the observed lactate signal is off-resonance pyruvate excitation likely enhanced by the rapid flow of the bolus.
Conclusion
Using simulation, we explored the dynamic nature of hyperpolarized agents in the vasculature and their impact on spectral spatial excitation. The primary effect is a reduction in the delivered excitation by up to 50% for reasonable arterial velocities. Additionally, flowing spins appear to be more sensitive to off-resonance excitation, allowing for some of the high concentration, high-velocity vascular pyruvate to appear in the lactate excitation. These signal impacts should be accounted for either during acquisition with improved pulses(4,8) or during data processing to ensure they do not affect signal quantification, with special care taken when measuring arterial input functions.Acknowledgements
This work was supported in part by the National Cancer Institute (R01CA211150), and GE Healthcare. The content is solely the responsibility of the authors and does not necessarily represent the official views of these agencies.References
1. Vaeggemose M, R FS, Laustsen C. Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application. Metabolites 2021;11(4).
2. Gordon JW, Chen HY, Autry A, Park I, Van Criekinge M, Mammoli D, Milshteyn E, Bok R, Xu D, Li Y, Aggarwal R, Chang S, Slater JB, Ferrone M, Nelson S, Kurhanewicz J, Larson PEZ, Vigneron DB. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med 2019;81(4):2702-2709.
3. Tropp J, Lupo JM, Chen A, Calderon P, McCune D, Grafendorfer T, Ozturk-Isik E, Larson PE, Hu S, Yen YF, Robb F, Bok R, Schulte R, Xu D, Hurd R, Vigneron D, Nelson S. Multi-channel metabolic imaging, with SENSE reconstruction, of hyperpolarized [1-(13)C] pyruvate in a live rat at 3.0 tesla on a clinical MR scanner. J Magn Reson 2011;208(1):171-177.
4. Fredrickson J, Meyer C, Pelc NJ. Flow Effects of Spectral Spatial Excitation. 1997; Vancouver. ISMRM. p 113.
5. Walker CX, Zhan Michel, Keith Martinez, Gary Harlan, Collin Gordon, Jeremy Carlon, Stephanie Williams, Sandra Gonzalez, Freddy Hash, Stacy Jones, Jerell McCoy, Asa Willis, Brandy Underwood, Michelle Day, Andrew Chariwala, Moin Le, Dao Waligorski, Gregory Vigneron, Daniel Schellingerhout, Dawid Lai, Stephen Bankson, James Imaging Treatment Response with Hyperpolarized Pyruvate in Anaplastic Thyroid Carcinoma. 2021. ISMRM.
6. Krejza J, Arkuszewski M, Kasner SE, Weigele J, Ustymowicz A, Hurst RW, Cucchiara BL, Messe SR. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006;37(4):1103-1105.
7. Lee W. General principles of carotid Doppler ultrasonography. Ultrasonography 2014;33(1):11-17.
8. Shin T, Hu BS, Nishimura DG. Off-resonance-robust velocity-selective magnetization preparation for non-contrast-enhanced peripheral MR angiography. Magn Reson Med 2013;70(5):1229-1240.
Figures