1876
On the importance of fetal brain numerical models for domain adaptation strategies in fetal brain MRI tissue segmentation1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Synopsis
Manual fetal brain tissue segmentation is needed for training machine learning methods but is a tedious and error-prone task. The generation of synthetic magnetic resonance images can overcome the lack of clinical annotations by supplementing scarce clinical fetal datasets. However, we highlight that the choice of the numerical model from which additional data are derived is key to maximize the segmentation accuracy of clinical data via domain adaptation strategies. We demonstrate that the resort to high-resolution segmented images from real neurotypical and pathological cases enhances the morphological variability compared to an atlas, resulting in improved fetal brain tissue delineation overall.
Introduction
In utero brain magnetic resonance imaging (MRI) is key to assessing fetal development. Clinical evaluation relies on fast spin echo (FSE) sequences and subsequent super-resolution (SR) reconstruction of a high-resolution (HR) volume of the fetal brain with reduced motion sensitivity1–4. Although laborious and prone to human error, manual annotation is needed for the training of supervised deep learning approaches that in turn enable automated delineation of fetal brain tissues5–7. As large publicly available fetal datasets remain scarce, the generalization across different domains has been barely explored8. Domain adaptation (DA) strategies can successfully address data distribution gaps, such as the use of different reconstruction pipelines, by generalizing the knowledge learned from a source domain to a target domain8,9. In this work, we further explore how the annotations of the fetal brain model are crucial to adapt the segmentation algorithm trained on an original clinical dataset to another dataset in a different domain.Methods
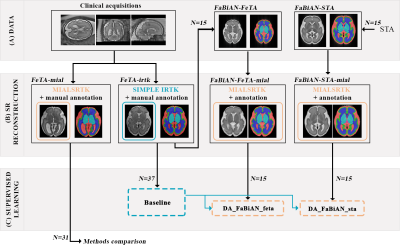
Figure 1 provides an overview of our methodological framework.Datasets. We include subjects from the Fetal Tissue Annotation Dataset (FeTA 2.0)7 that were reconstructed using either SIMPLE IRTK2 (source domain: FeTA-irtk) or MIALSRTK10 (target domain: FeTA-mial). Manual annotation of cerebrospinal fluid (CSF), cortical gray matter (GM), white matter (WM), cerebellum, deep GM (dGM) and brainstem in the SR volumes were refined in FeTA-mial subjects11. 37 FeTA-irtk subjects (17 neurotypical and 20 pathological subjects; gestational age (GA): 20.1-34.8 weeks) are used both for training and simulating clinically-relevant fetal brain MR images, and 31 FeTA-mial subjects (15 neurotypical and 16 pathological subjects; GA: 20.0-33.4 weeks) for evaluation.
Numerical models. FaBiAN, a Fetal Brain MR Acquisition Numerical phantom12,13, is used to simulate realistic T2-weighted single-shot FSE acquisitions from two sets of HR label maps:
- 15 subjects from a normative spatiotemporal MRI atlas (STA)14 (GA: 21-35 weeks);
- 15 clinical cases (eight neurotypical and seven pathological subjects, GA: 20.9-34.8 weeks) among the 37 FeTA-irtk subjects.
Whereas simulations based on STA rely on an averaged anatomy and same HR annotations for a given GA, using FeTA-irtk subjects as models of the fetal brain provides larger anatomical and annotation variability.
FaBiAN is set with acquisition parameters from the clinical protocol to scan FeTA subjects7,12: B0=1.5-3T; TE=116.928-121.904ms; TR=3000ms; excitation/refocusing pulse flip angles=90°/180°; slice thickness=3mm; field-of-view=240x240mm2-280x280mm2; reconstruction matrix: 512x512 voxels. For every subject, partially-overlapping orthogonal series of 2D thick slices (6.8±1.7) are generated with random little to moderate motion. Segmentation of the fetal brain model is propagated in all low-resolution series by a nearest-neighbour interpolation using FaBiAN simulation framework13. Fetal brain volumes are SR-reconstructed using the MIALSRTK BIDS App15, adapted to reconstruct associated label maps. The resulting FaBiAN-STA-mial and FaBiAN-FeTA-mial simulations aim at mimicking the target domain to support the generalization of a network trained on FeTA-irtk subjects to FeTA-mial subjects.
Configurations. The following 2D U-Net networks are trained:
● Baseline: trained on the full FeTA-irtk set.
● DA_FaBiAN_STA and DA_FaBiAN_FeTA: initialized with Baseline pre-trained weights to perform transfer learning using FaBiAN-STA-mial and FaBiAN-FeTA-mial respectively.
Evaluation. The network performance is evaluated with the Dice similarity coefficient (DSC)16. A paired Wilcoxon rank-sum test is performed between each experimental configuration, with Bonferroni multiple comparison correction for individual tissues (significance level = 0.05).
Results
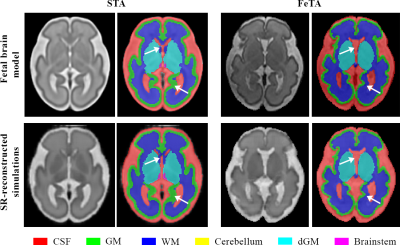
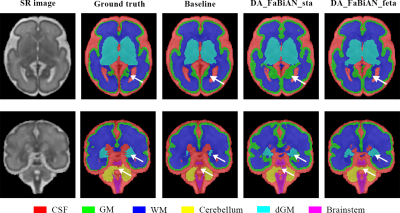
Figure 2 highlights areas of the fetal brain that are annotated in a significantly different way between STA14 and FeTA7, leading to two different models. Particularly, the cortical GM is thicker in the STA model, with a more accurate annotation of the deep sulci.Figure 3 qualitatively shows an overall more accurate segmentation using DA_FaBiAN_feta.
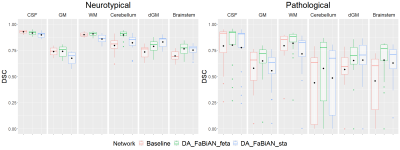
Figure 4 reports the mean DSC for each tissue in the different configurations studied, and Figure 5 displays the segmentation performance separated into neurotypical and pathological subjects. Overall, the performance of the segmentation algorithm is significantly improved when supplementing clinical data (Baseline) with synthetic, yet realistic MR images reconstructed in the target domain (DA_FaBiAN_X), especially with a fetal brain model close to the target domain (DA_FaBiAN_feta). DA_FaBiAN_feta provides statistically more accurate tissue segmentation than the Baseline except in the CSF where performances are only improved in pathological cases. Conversely, DA_FaBiAN_sta is less accurate in the CSF, the GM and the WM than the Baseline, whereas it outperforms all other configurations in the dGM. DA_FaBiAN_feta outperforms DA_FaBiAN_sta in the cerebellum, the brainstem, the WM and the GM. Improved segmentation of WM and GM is especially observed in pathological subjects.
Discussion
Originally, FaBiAN13 relies on a STA14 as underlying fetal brain model, resulting in smoothing of subtle inter-individual variability as atlas images are averaged over several subjects of the same GA. In this work, we show that simulating data from clinical HR MR images allows a segmentation network to better account for morphological variability between subjects of the same GA and to include pathological cases in the model.Conclusion
This work builds on a previous study that showed that complementing clinical datasets with synthetic MR images of the fetal brain significantly enhances the accuracy of multi-tissue segmentation9. Here, we further demonstrate the key role of consistent annotations of the fetal brain model from which synthetic MR images are derived. Future work will investigate the diversity of synthetic data generated in various domains for wider DA strategies.Acknowledgements
This work is supported by the Swiss National Science Foundation through grants 182602 and 141283. We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).References
1. Gholipour A, Estroff JA, Warfield SK. Robust super-resolution volume reconstruction from slice acquisitions: Application to fetal brain MRI. IEEE Trans Med Imaging. 2010;29(10):1739-1758. doi:10.1109/TMI.2010.2051680
2. Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012;16(8):1550-1564. doi:10.1016/j.media.2012.07.004
3. Tourbier S, Bresson X, Hagmann P, Thiran JP, Meuli R, Cuadra MB. An efficient total variation algorithm for super-resolution in fetal brain MRI with adaptive regularization. NeuroImage. 2015;118:584-597. doi:10/f7p5zx
4. Ebner M, Wang G, Li W, et al. An automated framework for localization, segmentation and super-resolution reconstruction of fetal brain MRI. NeuroImage. 2020;206:116324. doi:10.1016/j.neuroimage.2019.116324
5. Khalili N, Lessmann N, Turk E, et al. Automatic brain tissue segmentation in fetal MRI using convolutional neural networks. Magnetic Resonance Imaging. 2019;64:77-89. doi:10.1016/j.mri.2019.05.020
6. Delannoy Q, Pham CH, Cazorla C, et al. SegSRGAN: Super-resolution and segmentation using generative adversarial networks — Application to neonatal brain MRI. Computers in Biology and Medicine. 2020;120:103755. doi:10.1016/j.compbiomed.2020.103755
7. Payette K, de Dumast P, Kebiri H, et al. An automatic multi-tissue human fetal brain segmentation benchmark using the Fetal Tissue Annotation Dataset. Sci Data. 2021;8(1):167. doi:10.1038/s41597-021-00946-3
8. Payette K, Kottke R, Jakab A. Efficient multi-class fetal brain segmentation in high resolution MRI reconstructions with noisy labels. In: Hu Y, Licandro R, Noble JA, et al., eds. Medical Ultrasound, and Preterm, Perinatal and Paediatric Image Analysis. Lecture Notes in Computer Science. Springer International Publishing; 2020:295-304. doi:10.1007/978-3-030-60334-2_29
9. de Dumast P, Kebiri H, Payette K, Jakab A, Lajous H, Cuadra MB. Synthetic magnetic resonance images for domain adaptation: Application to fetal brain tissue segmentation. arXiv:211104737 [cs, eess]. Published online 2021. Accessed November 10, 2021. http://arxiv.org/abs/2111.04737
10. Tourbier S, Bresson X, Hagmann P, Meuli R, Bach Cuadra M. Sebastientourbier/Mialsuperresolutiontoolkit: MIAL Super-Resolution Toolkit v1.0. Zenodo; 2019. doi:10.5281/zenodo.2598448
11. Fidon L, Aertsen M, Emam D, et al. Label-set loss functions for partial supervision: Application to fetal brain 3D MRI parcellation. In: de Bruijne M, Cattin PC, Cotin S, et al., eds. Medical Image Computing and Computer Assisted Intervention – MICCAI 2021. Lecture Notes in Computer Science. Springer International Publishing; 2021:647-657. doi:10.1007/978-3-030-87196-3_60
12. Lajous H, Roy CW, Hilbert T, et al. FaBiAN: A Fetal Brain magnetic resonance Acquisition Numerical phantom. arXiv:210903624 [physics]. Published online 2021. Accessed October 4, 2021. http://arxiv.org/abs/2109.03624
13. Lajous H, Roy CW, Yerly J, Bach Cuadra M. FaBiAN v1.0. Zenodo; 2021. doi:10.5281/zenodo.5471095
14. Gholipour A, Rollins CK, Velasco-Annis C, et al. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep. 2017;7(1):1-13. doi:10.1038/s41598-017-00525-w
15. Tourbier S, De Dumast P, Kebiri H, Hagmann P, Bach Cuadra M. Medical-Image-Analysis-Laboratory/Mialsuperresolutiontoolkit: MIAL Super-Resolution Toolkit v2.0.1. Zenodo; 2020. doi:10.5281/zenodo.4392788
16. Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297-302. doi:10.2307/1932409
Figures