1872
Cortical thickness predicts pain sensitivity in a multi-centre cohort: a machine learning approach
Raviteja Kotikalapudi1,2, Balint Kincses1,2,3, Kevin Hoffschlag1, Matthias Zunhammer2, Tobias Schmidt-Wilcke4,5, Zsigmond T Kincses3, Ulrike Bingel2, and Tamas Spisak1,2
1Laboratory of Predictive NeuroImaging, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany, 2The Bingel Laboratory, Translational Pain Research Unit, University Hospital Essen, Essen, Germany, 3Department of Neurology, University of Szeged, Szeged, Hungary, 4Institute for Clinical Neurosciences and Medical Psychology, Heinrich Heine University, Dusseldorf, Germany, 5Neurocentre, District Hospital Mainkofen, Mainkofen, Deggendorf, Germany
1Laboratory of Predictive NeuroImaging, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany, 2The Bingel Laboratory, Translational Pain Research Unit, University Hospital Essen, Essen, Germany, 3Department of Neurology, University of Szeged, Szeged, Hungary, 4Institute for Clinical Neurosciences and Medical Psychology, Heinrich Heine University, Dusseldorf, Germany, 5Neurocentre, District Hospital Mainkofen, Mainkofen, Deggendorf, Germany
Synopsis
Individual sensitivity to pain is both a precursor and a symptom of many clinical pain conditions. A pain predictive model would have potential applications in objectively characterizing pain in acute and chronic pain individuals. Here, we developed a cortical thickness-based predictive model of pain sensitivity using a machine learning approach and multi-centre T1-weighted MRI and quantitative pain threshold data. We found that our model significantly predicts pain sensitivity, that was measured through heat, cold and mechanical stimuli. Furthermore, the predictions were exclusively driven by cortical thickness and not confounded by variables of demographic and psychological value.
Introduction
Individual’s sensitivity towards pain is manifested within the brain structure. However, it is not yet clear how this information can be used to build predictive models for pain sensitivity. In a multi-centre study, we aim at deriving a generalizable predictive model for estimating an individual’s pain sensitivity towards external noxious stimuli utilizing cortical thickness measures. The structure-based modelling of pain could be an important addition to the existing functional MRI-based neuromarkers of pain1,2, towards multi-modal pain prediction.Methods
At three different study sites, cumulatively 132 participants (mean age = 25 yrs., females = 65) were recruited. Pain ratings for heat, cold and mechanical pain thresholds were collected along with each participant’s T1-weighted MRI-scans. Using the pain ratings, a composite pain sensitivity measure i.e., QST (quantitative sensory testing) score was calculated. Cortical thickness measures were derived using the standard freesurfer pipeline and the multi-centre thickness measures were harmonized for site effects using ComBat3. A linear regression based LASSO model was fitted using the harmonized thickness measures and the QST scores, in a nested cross-validation framework (outer loop = inner loop = 10 cross-validations, figure 1). We performed model selection based on hyperparameter tuning values of the regularization rate α [ = 0.001, 0.01, 0.1, 1, 10]. Pain sensitivity scores were predicted with the LASSO model, and the estimates were also analyzed using mlconfound (https://github.com/pni-lab/mlconfound) for any potential confounding effects from additional measures including demographic (e.g. age, sex), psychological evaluations (e.g. anxiety, depression, sleep quality), and pain evaluating questionnaires (self-reporting pain questionnaires), which may influence pain sensitivity scores.Results
The best LASSO model was found to have a regularization factor α = 0.01. With this, the structure-based model explained 10% variance in pain sensitivity scores (r = .32, p < .000, figure 2). The final model utilizes cortical thickness of the rostral anterior cingulate cortex (rACC, r = -.38, p < .000), parahippocampal gyrus (PHG, r = -.24, p < 0.003) and temporal pole (TP, r = -.16, p < .03, figure 3). The predictions were not biased for any of the additional measures. It is important to note that the learning curve of the model saturates at approximately 50 samples, indicating that the study sample size is reasonable for deriving the predictive model.Discussion
Our LASSO-based framework predicted pain sensitivity in multiple centres using cortical thickness of three regions i.e. rACC, PHG and TP. Interestingly, we found the three regions to appear in all the nested cross-validations utilized in the model selection process. Due to the multi-center model development and the consistency in region selection by the model, our predictive approach can be expected to be generalizable towards external, unseen datasets as well. Secondly, our predictions are exclusively driven by brain morphology, since we did not find any significant correlations with potential confounders of demographic and psychological value. Most importantly, cortical thickness of rACC, PHG and TP are all negatively correlated in terms of estimating pain sensitivity. That means, a thicker cortex in these regions would lead to a lower pain sensitivity towards external noxious stimuli, which is in line with previous literature eliciting the role of these regions in pain4-7.Conclusion
The presented predictive model demonstrates that brain morphology holds a significant and clinically relevant predictive capacity for individual pain sensitivity differences. As an outlook, with a 10% explained variance for pain sensitivity, our model could serve as a computational biomarker based on structural morphology for explaining pain sensitivity, adding performance to the existing functional MRI-based modelling of pain2. Cortical thickness-based structural predictive modelling could extend our understanding of pain quantification in acute and chronic pain-related conditions. At the same time, our study significantly contributes to a multi-modal generalized framework (e.g., cortical thickness + fMRI + DTI) for future research that would implement predictive strategies in estimating pain sensitivity.Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 316803389 – SFB 1280 and TRR 289 Treatment Expectation - Projektnummer 422744262.References
- Wager, Tor D., Lauren Y. Atlas, Martin A. Lindquist, Mathieu Roy, Choong-Wan Woo, and Ethan Kross. 2013. “An fMRI-Based Neurologic Signature of Physical Pain.” The New England Journal of Medicine 368 (15): 1388–97.
- Spisak, Tamas, Balint Kincses, Frederik Schlitt, Matthias Zunhammer, Tobias Schmidt-Wilcke, Zsigmond T. Kincses, and Ulrike Bingel. 2020. “Pain-Free Resting-State Functional Brain Connectivity Predicts Individual Pain Sensitivity.” https://doi.org/10.1101/790709.
- Radua, Joaquim, Eduard Vieta, Russell Shinohara, Peter Kochunov, Yann Quidé, Melissa J. Green, Cynthia S. Weickert, et al. 2020. “Increased Power by Harmonizing Structural MRI Site Differences with the ComBat Batch Adjustment Method in ENIGMA.” NeuroImage 218 (September): 116956.
- Grant, Joshua A., Jérôme Courtemanche, Emma G. Duerden, Gary H. Duncan, and Pierre Rainville. 2010. “Cortical Thickness and Pain Sensitivity in Zen Meditators.” Emotion 10 (1): 43–53.
- Bingel, U., E. Schoell, W. Herken, C. Büchel, and A. May. 2007. “Habituation to Painful Stimulation Involves the Antinociceptive System.” Pain. https://doi.org/10.1016/j.pain.2006.12.005.
- Spisák, Tamás, Zsófia Pozsgay, Csaba Aranyi, Szabolcs Dávid, Pál Kocsis, Gabriella Nyitrai, Dávid Gajári, Miklós Emri, András Czurkó, and Zsigmond Tamás Kincses. 2017. “Central Sensitization-Related Changes of Effective and Functional Connectivity in the Rat Inflammatory Trigeminal Pain Model.” Neuroscience 344 (March): 133–47.
- Apkarian, A. Vania, M. Catherine Bushnell, Rolf-Detlef Treede, and Jon-Kar Zubieta. 2005. “Human Brain Mechanisms of Pain Perception and Regulation in Health and Disease.” European Journal of Pain 9 (4): 463–84.
Figures
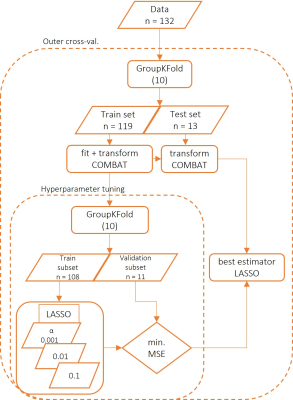
Block diagram for the predictive modelling of pain sensitivity.

A regression scatter plot for predicted versus actual pain sensitivity (QST score) is presented here (a). Also shown is the learning curve for the model, which saturates at approximately 50 samples (b).
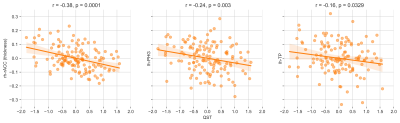
The three regions that predict pain sensitivity are shown here i.e. right rACC, left PHG and left TP. All the three regions are negatively associated with pain sensitivity.
DOI: https://doi.org/10.58530/2022/1872