1869
Comparison of Imaging Methods for Fast Whole-Body MRI Screening1Physics, Clatterbridge Cancer Centre, Liverpool, United Kingdom, 2Radiology, Clatterbridge Cancer Centre, Liverpool, United Kingdom
Synopsis
Improvements in image quality and scan time coupled with AI reading of images, is enabling the possibility of quickly screening all patients who attend MRI for malignant tumours. This research explores the imaging and clinical requirements for such a programme, and reports on the ability of various sequences and patient configurations to meet those requirements.
Introduction
MR whole-body screening has been identified as potentially useful for early diagnosis of a number of conditions [1,2]. Recent advances in MRI technology and AI processing enable the possibility of fast whole-body imaging and processing in real-time, reducing the cost of implementing a screening program for all attending patients, potentially increasing patient care for minimal outlay [3].Two crucial aspects of this to be addressed are the fast acquisition of suitable data without disrupting the clinical workflow, and the processing of the images to find suspected tumours.
Here we report on the first steps of implementing such a system, comparing different imaging methods for fast whole body acquisitions; including an assessment of various sequences and patient setups to cover the anatomy, tackle motion artefacts, and maintain resolution and signal levels sufficient for clinical interpretation.
Methods
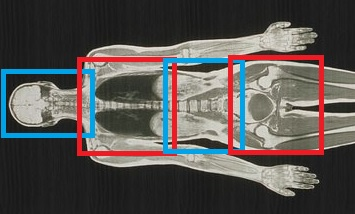
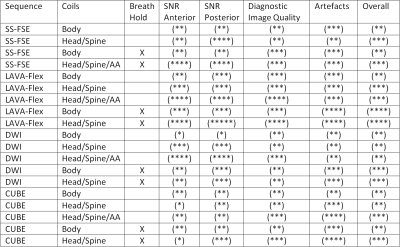
Fourteen volunteers (6 male, 8 female, BMI range 18-27) were imaged on a GE (General Electric, USA) MR450W 1.5T scanner at the Clatterbridge Cancer Centre. As shown in FIgure 1, the scanner was configured to move the table through 4 overlapping stations (head/neck, torso, abdomen, pelvis). Images were acquired axially with the in-built body coil, and subsequently with the spine with head/neck coils, with and without anterior array elements. Image SNR was analysed across the patient anatomy, as well as general image quality and artefacts were qualitatively recorded.The following pulse sequences were acquired: Single-Shot FSE, LAVA Flex (2-point Dixon), CUBE (3D FSE), and DWI with a single b-value of 800.
Typical spatial parameters of 38cm FOV, 80 transverse slices of 5mm slice thickness and 2mm slice gap, and an acquistion matrix size of 256x128 at each station. To account for different sized patients the slice gap was modified along with the number of slices where necessary. A smaller FOV of 24cm was used at the head/neck station.
Breath holding as well as free breathing was used when scanning at the central two stations.
Image sets were also assessed by a radiologist for their diagnostic quality and any incidental findings were reported.
Results
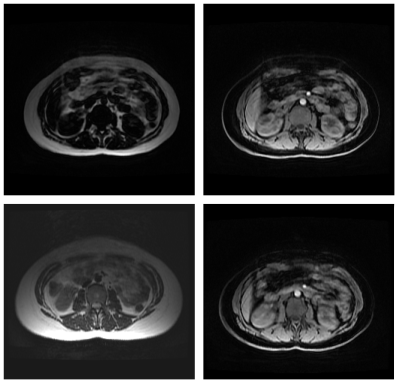
As seen in Figures 2 and 3, the image quality varied across different setups and as expected was significantly lower for all sequences when compared with standard diagnositic quality images. Anterior image quality was found to be barely acceptable without the anterior array coil, yet it was still found to be useful for visualisation of important anatomical details on all but the largest patients. Breath holding was found to be superior to free breathing in all cases, free breathing also caused smaller artefacts in the pelvis.Scan times per station were below 23 seconds for all sequences, and the minimum examination time for a full set of LAVA Flex data was 2:19 including table movements and shimming.
Initial experience showed that setup time of the volunteers would be no longer than usual for head and spine coils only, compared with around 1 minute longer for setting up an additional anterior array coil. For complete coverage of larger patients, two array coils were necessary.
Discussion
Although this project is in its early stages, it has already demonstrated the acquisition of screening images of reasonable quality in the majority of subjects without using anterior array elements. This is considered an important constraint of the project since it will not require significant changes in working practices, or patient setups, and therefore may be introduced as a complimentary localiser that will enable wider acceptance in clinical departments, increasing the potential amount of data collected and thus helping to improve the performance of the machine learning algorithm.Examination of the LAVA-Flex images showed that they were capable of interpretation by radiologists, and indeed 36% of the exams raised incidental findings all of which were indicated by the LAVA-Flex sequence. This raises ethical questions which have been previously discussed by Morin [4]. It is apparent that while Dixon Imaging also provides additional contrast data that will likely be beneficial for an AI image processor, it also potentially displays unique artefacts such as the fat-water swapping as seen in FIgure 2. The AI image processing will be a key component of this project and various schemes of data pre-processing and SVM and neural network architecture are currently under investigation that may help improve data quality and ultimately reduce artefacts [5,6]. It is expected that anonymised patient meta data such as age, sex, BMI and clincal diagnosis will also aid this process. This may require further modification of the sequences acquired as the system develops.
Conclusion
This work provides a basis on which a future MRI screening program may be implemented without significant extra cost to patients or imaging departments. The next stage will include the scanning of patients and the development of the sequence to include automatic table movement and incorporate respiratory gating. Coupled with the larger data set, this will then lead to the development and training of the AI system to recognise tumours in low quality MR images.Acknowledgements
This research is funded by CRFS under grant number 191056.References
[1] Hegenscheid K, Kühn JP, Völzke H et al: Whole-body magnetic resonance imaging of healthy volunteers: pilot study results from the population-based SHIP study. Rofo, 2009; 181: 748–59.
[2] Tarnocki DL et al., Clinical Value of whole-body MRI in health screening of general adult population, Radiol Oncol 2015 Mar; 49(1): 10-16.
[3] Ladd SC, Whole-body MRI as a screening tool? Eur J Radiol, 2009; 70: 452–62.
[4] Morin SH, Cobbold JF, Lim AK et al: Incidental findings in healthy control research subjects using whole-body MRI. Eur J Radiol, 2009; 72: 529–33.
[5] Suzuki K. Pixel-based machine learning in medical imaging. Int J Biomed Imaging, 2012; 792079.
[6] Erickson, B.J., Korfiatis, P., Akkus, Z., Kline, T.L. Machine learning for medical imaging. Radiographics. 2017; 37:505–515.
Figures