1860
Radial Echo-Planar Imaging with Subspace Reconstruction for Brain MRI1Institute for Diagnostic and Interventional Radiology, University Medical Center Goettingen, Goettingen, Germany, 2Institute of Medical Engineering, Graz University of Technology, Graz, Austria
Synopsis
While echo planar imaging (EPI) is successful in many MRI applications, this study demonstrates that radial EPI is applicable to brain MRI as well. Initial volumetric brain experiments at 1 mm isotropic resolution show that radial EPI is capable of rendering high-quality T1- and T2*-weighted images at 2.5 and 1.3 minutes scan time, respectively. Further, radial EPI requires neither magnetization preparation nor fat saturation.
INTRODUCTION
Echo-planar imaging (EPI) 1 and its varinat EPTI 2, a breakthrough rapid MR imaging technique, reads out multiple echoes per RF excitation. Given its fast $$$k$$$-space coverage and $$$T_2^*$$$ sensitivity, EPI has been successful in functional, diffusion and quantitative MRI. However, EPI is challenging due to the use of reversed readout polarities between echoes and susceptibility artifacts induced by long readout time. Therefore, this work aims to explore the applicability of radial EPI 3,4, as an alternative to the standard Cartesian EPI, for brain MRI.METHODS
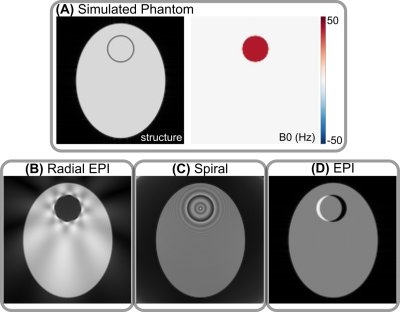
Numerical Simulations of Single-Shot Radial EPI, Spiral, and Cartesian EPIA numerical simulation framework 5,6 based on analytical FFT has been implemented to study single-shot acquisitions with the existence of static off-resonance. As shown in Figure 1, different single-shot trajectories lead to their corresponding characteristic off-resonance artifacts with NUFFT 7 or FFT reconstructions. Radial EPI shows signal void and streaks due to the overlap of all echoes in the $$$k$$$-space center; Single-shot center-out spiral acquisition shows ripple-like artifacts; Cartesian EPI shows spatial distortion due to phase shifts along the phase-encoding direction. These off-resonance artifacts can be removed with $$$B_0$$$-informed iterative reconstruction based on the conjugate gradient method (results not shown).
Segmented Radial EPI
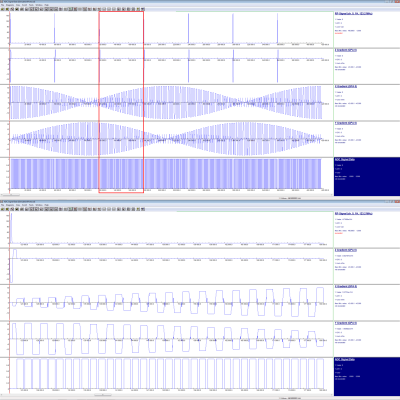
The segmented radial EPI sequence we implemented is displayed in Figure 2. In the $$$k_x$$$-$$$k_y$$$ plane, multiple echoes with different radial angles are sampled per RF excitation with the use of blip gradients. This set of echoes forms one segment in $$$k$$$-space, and is repeated for all $$$k_z$$$ partitions in stack-of-stars acquisition 8. The subsequent excitation starts the sampling of the next segment.
Brain MRI Experiments
All experiments were conducted at 3 T (Skyra, Siemens Healthineers, Erlangen, Germany), with a 20-channel head/neck coil. Three volunteers with written consent participated in the brain experiments. Two types of scans were performed:
- Ultrafast whole brain $$$T_1$$$-weighted scan with 1 mm isotropic resolution and without magnetization preparation. Detailed imaging parameters are: flip angle 12 degree, bandwidth 840 Hz/pixel, FOV 220 mm, matrix size 220 x 220 x 192, 125 excitation per partition, 3 echoes per excitation with TE 1.7, 3.2, 5.7 ms and TR 6.4 ms. Total scan time is 2.5 minutes. Images were reconstructed with density-compensated NUFFT 7.
- Ultrafast whole brain $$$T_2^*$$$-weighted scan with 1 mm isotropic resolution. Detailed imaging parameters are: flip angle 4 degree, 7 excitation per partition, 35 echoes per excitation with TE ranging from 1.70 to 55.7 ms and TR 57.4 ms. Total scan time is 1.3 minutes. The other parameters are the same as the first protocol. Images were obtained via local low-rank constrained subspace reconstruction in BART 9.
RESULTS
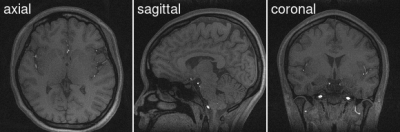
Rapid whole brain $$$T_1$$$-weighted scan with 1 mm isotropic resolution and without magnetization preparationWith the efficient and rapid triple-echo stack-of-stars FLASH technique, high-quality images are obtained with good $$$T_1$$$ contrast. Specifically, we observe clear boundary between white and gray matters, and the CSF region appears dark. Although promising, future work would be required to optimize imaging parameters for better $$$T_1$$$-weighted rapid brain scan.
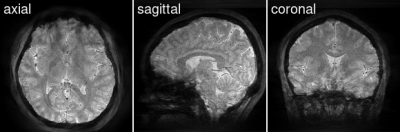
Rapid whole brain $$$T_2^*$$$-weighted scan with 1 mm isotropic resolution
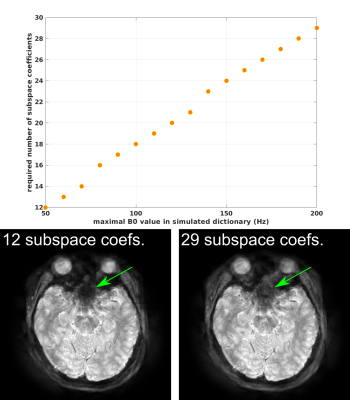
Similar to EPI, the proposed radial EPI sequence is well suited for $$$T_2^*$$$-weighted brain imaging with ultra short scan time. Residual streaking artifacts are still visible in regions with large static $$$B_0$$$ inhomogeneity. The reason may be the limited range of $$$B_0$$$ values in the dictionary and the small number of subspace coefficients, which are insufficient to capture the full $$$B_0$$$ inhomogeneity range. As show in Figure 5, larger $$$B_0$$$ range in the simulated signal requires more subspace coefficients to closely represent the full dictionary.
DISCUSSION & CONCLUSION
This work demonstrates the applicability and efficiency of the radial EPI sequence for brain MRI. Initial experiments focused on rapid $$$T_1$$$- and $$$T_2^*$$$-weighted scans. First, we did not employ any magnetization preparation pulse for the $$$T_1$$$-weighted scan, but the resulting $$$T_1$$$ contrast looks acceptable. The total acquisition time is rather short. The reason is that for the specific TR required for good $$$T_1$$$ contrast, multi-echo acquisition is used such that no time is wasted before TR. Second, radial EPI requires no fat saturation and shows no chemical-shift spatial shift artifact. This is because in radial EPI all echoes pass through $$$k$$$-space center, which results in intrinsic fat saturation via "phase cancellation" effect.$$$B_0$$$ field inhomogeneity causes image artifacts. To alleviate this problem, both $$$B_0$$$-informed reconstruction and subspace reconstruction are feasible. The former requires either a joint estimation or a calibrated $$$B_0$$$ field map. The latter approach instead reconstructs linear subspace coefficients. However, care must be taken on the selection of $$$B_0$$$ range in the dictionary and the coefficient number. Although larger subspace coefficients may be beneficial to capture fast $$$B_0$$$ field change, it requires longer computation time during the iterative subspace reconstruction. Therefore, as a next step, it would be logical to explore an appropriate reconstruction for $$$T_2^*$$$-weighted radial EPI scan for broader clinical applications.
Acknowledgements
The authors would like to thank German Research Foundation (DFG) for the support of the project 427934942.References
[1] Mansfield P. Multi planar image formation using NMR spin echoes. J Phys C 1977;10:L55-L58.
[2] Dong Z, Wang F, Chan KS, Reese TG, Bilgic B, Marques JP, et al. Variable flip angle echo planar time-resolved imaging (vFA-EPTI) for fast high-resolution gradient echo myelin water imaging. NeuroImage 2021;232.
[3] Sliva AC, Barbier EL, Lowe IJ, Koretsky AP. Radial echo-planar imaging. J Magn Reson 1998;135:242-247.
[4] Tan Z, Voit D, Kollmeier JM, Uecker M, Frahm J. Dynamic water/fat separation and B0 inhomogeneity mapping -- Joint estimation using undersampled triple-echo multi-spoke radial FLASH. Magn Reson Med 2019;82:1000-1011.
[5] Koay CG, Sarlls JE, Özarslan E. Three-dimensional analytical MRI phantom in the Fourier domain. Magn Reson Med 2007;58:430-436.
[6] Guerquin-Kern M, Lejeune L, Pruessmann KP, Unser M. Realistic analytical phantoms for parallel MRI. IEEE Trans Med Imaging 2012;31:626-636.
[7] Fessler JF. https://github.com/JeffFessler/mirt.
[8] Block KT, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J Korean Soc Magn Reson Med 2014;18:87-106.
[9] Uecker M. https://github.com/mrirecon/bart.
Figures