1854
MRI of individuals with cochlear implants: surgical planning, current practice, success rates, and stakeholder opinions1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Hearing Sciences, Division of Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 3Hearing Theme, NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom, 4Imaging Theme, NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom, 5Radiological Sciences, Division of Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 6Philips Healthcare, Best, Netherlands, 7Radiology Department, Queens Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom, 8National Acoustic Laboratories, Sydney, Australia
Synopsis
A cochlear implant (CI) contains a magnet implanted under the scalp. MRI of CI users is associated with safety concerns, significant discomfort, and image distortion. A CI placed under a swimming cap is a feasible tool for observing the effect of CI location on image usability within a single subject and potentially informing surgical planning. 35-70% of radiological features in the head were deemed unaffected by the implant. Online survey results highlight the need for consistent publication of clear, succinct, and standardised information for healthcare professionals and CI users. CI user consultation is scarce, meaning their views are often neglected.
Introduction
A cochlear implant (CI) partially restores hearing to deaf individuals. A CI contains an implanted magnet and electronic receiver/stimulator placed under the scalp. Deaf children generally receive a CI in the first year of life and thus are likely to require post-CI-implantation MRI during their lifetime (for either clinical diagnostic or research purposes). Implanted magnets raise safety concerns around MRI of any anatomical region in CI users due to the risk of severe discomfort and ultimately displacement of the implant magnet while in proximity to the scanner. Displacement can cause soft-tissue damage that, while healing, means the CI cannot be used [1]. Accordingly, MRI is often avoided, in favour of other imaging modalities that are less diagnostically powerful and/or use ionising radiation (e.g. CT). MRI of the head is further confounded by image distortion caused by the implant [2]. Recent advancements in implant technology have sought to improve safety and comfort, but image quality remains problematic. The heterogeneity of devices in use is high and increasing. We aimed to improve how MRI is conducted in CI users through three related studies.Three studies were conducted:
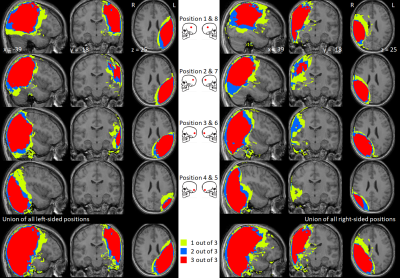
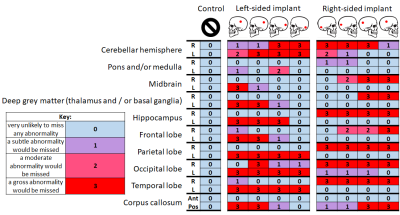
- An MRI study of three healthy volunteers wearing a non-functioning CI with new-generation rotating magnet placed underneath a swimming cap. The study was risk assessed for safety and feasibility. Imaging was conducted to characterise the extent and distribution of the CI artefact with respect to clinical image interpretation and to observe the effect of implant placement and anatomical variation on image usability. Individuals underwent brain MRI using a pre-set T1-weighted sequence: steady-state (fast-field echo; FFE) gradient-echo acquisition; 1.5 mm isotropic reconstructed to 1 mm isotropic resolution; FOV 240×240×160 mm3; TE 1.52 ms; TR 25 ms, flip angle 30°; bandwidth 285 Hz; SENSE factor 1.6; 150 contiguous sagittal slices provided whole-head coverage; SAR 0.484 Wkg-1, duration 2 min 57 s. Nine repeat volumes were acquired in each person with the CI placed at four plausible scalp positions on each side (as informed by an experienced CI surgeon), and without the CI in situ. The extent of the image artefact was assessed quantitatively using voxel-based techniques. Two radiologists independently rated the likely impact of the artefact on the detection of pathology for 20 anatomical brain locations.
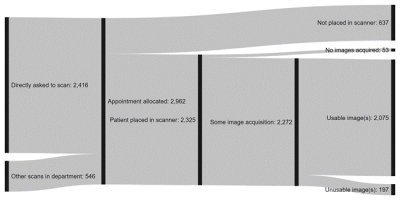
- A global online survey of 237 English-speaking healthcare professionals working in MRI sought to characterise practice and opinions around the current clinical use of MRI in CI users, to quantify typical success rates for clinical MRI in patients with CIs, and identify the main bottlenecks where patients are lost from the process.
- A global online survey of 310 English-speaking CI users was conducted to elicit opinions and beliefs around the hypothetical eventuality of needing to undergo MRI.
The results of the three studies were:
- There was statistically substantial inter-rater agreement, confirmed by Quadratic Weighted Cohen’s κ values of 0.70, 0.72, and 0.78 for each participant, respectively. The likelihood of missing pathology was associated significantly with signal dropout, which occurred 30% of the time (r=0.71, p<0.05), signal pileup (prevalence=26%; r=0.59, p<0.05), banding (prevalence=12%; r=0.29, p<0.05), and distortion (prevalence=2%; r=0.07, p<0.05). The presence of rippling (prevalence=16%) was not found to be strongly correlated with the likelihood of missing pathology (r=0.03). Detection of pathology in contralateral structures and the anterior corpus callosum was rarely affected by artefacts (Figures 1 and 2). Postero-inferior CI locations selectively spared ipsilateral midbrain, deep grey matter, and frontal lobes.
- Approximately 75% of CI users referred for an MRI proceeded to image acquisition, of which ~70% of cases comprised image acquisition on the head and the remaining cases on another area. The proportion of these images that were usable was 93% and 99%, respectively (Figure 3). Confidence in most processes was high, with at least two-thirds of respondents reporting to be very or somewhat confident in obtaining consent and acquiring images. Conversely, fewer than half the respondents had the same confidence when splinting and bandaging the implant and troubleshooting any issues arising. Patient safety was rated of paramount importance, with patient comfort a clear second and image quality third.
- 55% of respondents had been told whether their CI model could undergo MRI. 31% considered MRI when deciding whether to receive a CI and 28% when deciding which CI model to have. 59% would consider minor surgery to upgrade their retaining magnet to one of a rotating design.
Conclusions and discussion
- A CI placed under a swimming cap is a feasible tool for observing the effect of CI location on image usability within a single subject and potentially informing surgical planning. Regardless of CI placement, artefacts involving ipsilateral parietal, temporal, and occipital lobes severely limit the diagnostic utility of images acquired, however between 35% and 70% of radiological features in the head were deemed unaffected by the implant.
- The results from the two surveys highlight the need for consistent publication of clear, succinct, and standardised information aimed at both healthcare professionals and CI users. Radiographers require unambiguous operating procedures for scanning patients with CIs, together with regular training on how to safely and effectively scan the vast array of CIs used by adults and children around the world. CI user consultation of this sort is scarce, meaning the views of CI users are often neglected.
Acknowledgements
This work was supported by the National Institute for Health Research Nottingham Biomedical Research Centre. The design of the cochlear implant user survey was supported by the Institute of Physics.References
1. Walton J, et al. MRI without magnet removal in neurofibromatosis type 2 patients with cochlear and auditory brainstem implants. Otol Neurotol. 2014 Jun;35(5):821-5.
2. Edmonson HA, et al. MR Imaging and Cochlear Implants with Retained Internal Magnets: Reducing Artifacts near Highly Inhomogeneous Magnetic Fields. Radiographics. 2018 Jan-Feb;38(1):94-106.
Figures