1852
Identification of a single-dose, dual-echo based CBV threshold for fractional tumor burden mapping in recurrent glioblastoma1Division of Neuroimaging Research and Barrow Neuroimaging Innovation Center, Barrow Neurological Institute, Phoenix, AZ, United States, 2Mayo Clinic Arizona, Phoenix, AZ, United States, 3Southwest Neuroimaging at Barrow Neurological Institute, Phoeix, AZ, United States, 4Genentech, South Francisco, CA, United States
Synopsis
Reliable differentiation of tumor recurrence and treatment effects in glioblastoma patients is still a prevailing challenge. The purpose of this study is to identify the optimal threshold for generating fractional tumor burden (FTB) maps derived from single-dose, dual-echo based standardized relative cerebral blood volume measurements. To establish the threshold, dual-echo data was compared to the well-validated, double-dose single echo standardized FTB map. In summary, with the optimized threshold, the single-dose, dual-echo approach yielded FTB maps that strongly agreed with the reference standard, providing a compelling option for clinical use.
Introduction
Dynamic susceptibility contrast (DSC) MRI measures of relative cerebral blood volume (RCBV) can differentiate glioma grades, distinguish tumor recurrence from post-treatment radiation effects (PTRE), reflect relevant molecular and genetic drivers of tumor status, and predict treatment response and patient survival. In particular, image-localized biopsies and spatially matched DSC-MRI have been used to identify an RCBV threshold that accurately separates recurrent tumor samples from those with PTRE1. Quantifying the fraction of tumor voxels relative to PTRE voxels defines the Fractional Tumor Burden (FTB) metric, which correlates with local histologic tumor burden2. With the availability of FDA-cleared software2, FTB mapping is increasingly used in clinical practice.Prior FTB validation efforts have primarily focused on RCBV maps derived from the well-established single-echo, double-contrast agent dose DSC-MRI acquisition protocol. Recently, a single dose, dual-echo RCBV protocol was clinically validated by direct comparison to the double dose standard4. As this protocol enables half the contrast agent dose and only a single injection, there is a compelling need to establish dual-echo-based FTB mapping. In this study, we acquired dual-echo DSC-MRI data followed by the reference single-echo DSC-MRI data so that we could establish, at the voxel-wise level, the optimal rCBV threshold for dual-echo based FTB mapping.
Methods
This ongoing study currently includes sixteen glioblastoma patients undergoing perfusion scans to confirm suspected tumor recurrence. Dual-echo and single-echo DSC-MRI perfusion data were acquired during two sequential contrast agent injections. Scan parameters for the dual-echo scan were TR= 0.5s, TE1/TE2 = 7.4/33.6 s, flip angle = 75º. The double-dose, single echo scan parameters matched recent recommendations3.Standardized RBCV (sRCBV) maps were employed as they do not require user-defined reference regions and were recently validated for FTB mapping2. The double dose, single-echo (reference) FTB maps were generated using the clinical software, IB Rad Tech (Imaging Biometrics, Elm Grove, WI) which defines three categories: PTRE (sRCBV < 1), admixture (sRCBV: 1 - 1.55), and tumor recurrence (sRCBV > 1.55). Dual-echo-based sRCBV maps were computed using IB Neuro. To assess sRCBV agreement between acquisition protocols, the concordance correlation coefficient (CCC) was computed between the mean tumor sRCBV values across all patients. Next, a multiclass receiver operating characteristics (ROC) analysis was performed in MATLAB to identify the optimum sRCBV threshold, wherein dual-echo-based FTB maps, generated across a range of thresholds, were compared with the reference FTB maps.
Results/Discussions
Figure 1 shows the mean sRCBV comparison across the patients for the single-dose, dual-echo and corresponding double-dose, single-echo data. Consistent with the prior study4, there is strong agreement between the two protocols (CCC = 0.95).Figure 2 shows the receiver operating characteristics curve used to differentiate tumor and PTRE. The ROC analysis provided optimal sRCBV thresholds of 1 and 1.65 for identifying PTRE and tumor voxels, respectively. For comparison, IB Rad Tech uses thresholds of 1 and 1.55. Across all patients, the resulting dual-echo FTB maps have an AUROC = 0.90/0.92 (sensitivity = 82.3%/78.1%, specificity = 84.7%/92%) for PTRE and tumor, respectively. An example reference FTB map generated using IB Rad Tech (right) and the corresponding single-dose, dual-echo FTB map (left) is shown in Figure 3. Visually, the two FTB maps are in strong agreement, each identifying the same regions as either recurrent tumor or PTRE.
Conclusion
We have identified the optimum thresholds of 1 and 1.65 for the single-dose, dual-echo sRCBV to differentiate tumor and PTRE. While the prior study validated agreement of mean sRCBV values between the protocols4, this study confirmed that their voxel-wise agreement is suitable for reliable FTB mapping. Dual-echo-based DSC-MRI acquisitions enable robust single-dose RCBV and FTB mapping, provide pulse sequence parameter flexibility4, and should improve reproducibility by mitigating variations in preload dose and incubation time.Acknowledgements
NIH/NCI 2R01 CA158079-01References
[1] Leland S. Hu, Jennifer M. Eschbacher. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-Oncol. 2012; 14(7): 919-30.
[2] J.M. Hoxworth, J.M. Eschbacher, A.C. Gonzales. Performance of Standardized Relative CBV for Quantifying Regional Histologic Tumor Burden in Recurrent High-Grade Glioma: Comparison against Normalized Relative CBV Using Image-Localized Stereotactic Biopsies. AJNR 2020; 41(3): 408-415.
[3] Jerrold L Boxerman, Chad C Quarles, Leland S HuConsensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro-Oncol. 2020; 22(9): 1262-1275.
[4] Ashely Stokes, Maurizio Bergamio, Lea Alhilali. Evaluation of single bolus, dual-echo dynamic susceptibility contrast MRI protocol in brain tumor patients. JCBFM 2021.Figures
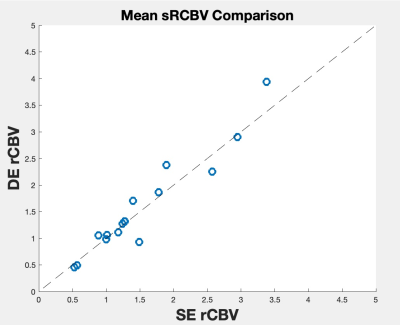
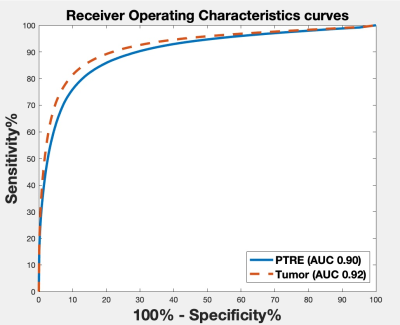
Figure 2: Receiver Operating Characteristics curve for the tumor and PTRE. The AUROC for tumor and PTRE are 0.92 and 0.90 respectively.
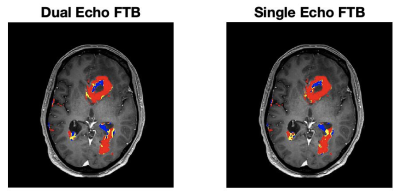
Figure 3: An example FTB map generated using the reference double-dose, single-echo DSC-MRI protocol and the IB Rad Tech clinical software (left) and derived from the application of the identified optimal threshold to single-dose, dual-echo sRCBV maps (right). Blue represents areas of PTRE, red represents areas of recurrent tumor, and yellow as admixture.