1850
Retrospective motion correction in multiple average, free-breathing cardiac cine imaging
Alexander Paul Neofytou1, Radhouene Neji1,2, James Wong3, Anastasia Fotaki1, Joana Ferreira1, Carl Evans1, Filippo Bosio1, Nabila Mughal1, Reza Razavi1, Kuberan Pushparajah1, and Sébastien Roujol1
1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Department of Paediatric Cardiology, Evelina London Children’s Hospital, London, United Kingdom
1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Department of Paediatric Cardiology, Evelina London Children’s Hospital, London, United Kingdom
Synopsis
A novel reconstruction technique based on iterative rejection of segmented k-space was developed for retrospective correction of respiratory motion in multiple average, free-breathing cine images. In comparison to standard signal averaging reconstruction, it provides higher sharpness, SNR, CNR, image quality, and rate of diagnostic quality images.
Introduction
Cardiac cine imaging is an essential technique in clinical cardiac magnetic resonance (CMR) examinations and is used for the analysis of ventricular structures and function1. Cine images are normally acquired within a breath-hold (1-2 slices/breathhold) using a segmented bSSFP acquisition over 10-12 cardiac cycles. Unfortunately, images are severely degraded in patients unable to sustain stable breathholds such as patients with impaired lung function or patients less cooperative in following instructions (e.g. the paediatric population). A way to alleviate this is to allow patients to breathe freely and acquire multiple signal averages (typically three), which helps minimise motion artefacts seen in the reconstructed images. However, free-breathing acquired images with multiple averages can sometimes suffer from important respiratory motion artefacts and be of non-diagnostic quality. This study sought to develop a novel motion-robust reconstruction technique for free-breathing, multiple average, cine acquisition.Methods
Proposed reconstruction:The proposed reconstruction is summarised in Figure 1 for a typical acquisition with three signal averages. This technique relies on the iterative removal of k-space segments that contribute the most to motion corruption/blurring. An image sharpness or focus measure (image gradient energy2) was used as a surrogate for quantifying the level of motion corruption/blurring in the cine images (i.e. the more blurry the image, the lower the focus). At each iteration, the k-space segment across the three corresponding k-space matrices that outputted the highest increase in focus, when removed, was removed. This process was repeated until convergence of the image focus. A condition was set such that across the three signal averages, one segment out of the respective set of three segments needed to remain to ensure initial k-space under-sampling was preserved. To mitigate the potential loss of SNR/CNR associated with the removal of k-space segments, this reconstruction was performed three times using different initial conditions (removal of 2 out of 3 central segments) to generate three images. On a slice-by-slice basis, the image series with the highest mean focus (measured across cardiac phases) was labelled as the sharpest image series (Isharpest). The remaining two series were co-registered to Isharpest using non-rigid image registration3. An average image of these three co-registered series was generated as the final reconstruction (Icombined3).
Evaluation:
Segmented balanced steady-state free precession (bSSFP) cine images (short-axis stack) were acquired free-breathing using retrospective ECG gating in 15 patients undergoing routine CMR examination. Imaging parameters were as follows: TE/TR=~1.2/2.8ms, Flip angle=52°, FOV=~225×300mm2, Acq.voxel size=~2.2×1.6mm2, slice thickness=8mm, BW=930Hz/px, GRAPPA factor=2, NSA = 3, temporal resolution=~40ms, no. slices=15. Images were acquired at 1.5T (MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany). All data were exported offline and three reconstructed datasets were generated in Matlab for each scan: 1) standard approach/signal averaging (Istandard), 2) intermediate reconstruction (Isharpest) and 3) proposed reconstruction (Icombined3). Qualitative image assessment (0 = non-diagnostic, 1 = diagnostic (sub-optimum image quality - presence of motion artefacts/blurring), 2 = diagnostic (excellent image quality - no motion artefacts/no blurring/sharp myocardial edges)) was performed by consensus of two experienced CMR readers on the three reconstruction techniques. The CMR readers were blinded from the clinical details of the patients and the reconstruction method (presented in a randomised order). Quantitative image analysis involving septal blood-myocardium sharpness4, signal-to-noise ratio (SNR) of blood and myocardium and their respective contrast-to-noise ratio (CNR) were also performed.
Results
Example images reconstructed from two patients using the three techniques is shown in Figure 2 and demonstrate the improved sharpness and image quality achieved with both the “Sharpest” and “Combined3” reconstructions. Over all patients, septal blood-myocardium sharpness increased significantly (Figure 3a) in “Sharpest” (0.79±0.09) and “Combined3” (0.79±0.1) in comparison to “Standard” (0.74±0.12, p=0.004 & p=0.04, respectively). Blood SNR/myocardial SNR in “standard” (94±30/33±10) was higher than in “Sharpest” (80±25, p=0.002/28±8, p=0.005) and tend to be lower than in “Combined3” (105±33, p=0.02/36±12, p=0.06) (Figure 3b-c). Similarly, blood/myocardial CNR in “Standard” (61±22) was higher than in “Sharpest” (53±19, p=0.003) and lower than in “Combined3” (69±24, p=0.007). Image quality scores obtained with “Sharpest” (1.8±0.2) and “Combined3” (1.9±0.2) were higher than in “Standard” (1.6±0.4, p=0.02 & p=0.008, respectively). 94% and 99% of slices were of diagnostic value in “Standard” and “Sharpest”, respectively, while 100% of slices were of diagnostic value in “Combined3” (Figure 4).Discussion
The proposed reconstruction results in higher image quality and diagnostic rate, which may prevent the need for sedation or repeat patient scanning. Evaluation in a larger cohort of patients is now warranted. The motion correction algorithm is currently computationally expensive (~1 hour to reconstruct one short axis stack). Dedicated GPUs and parallel computing could be used to alleviate this. The method was demonstrated using free-breathing CINE images acquired with three signal averages, which is typically used for free-breathing CINE imaging. However, this technique can in theory be generalised to any number of signal averages. This technique may also be valuable for other sequences acquired under free-breathing conditions with multiple averages, such as for flow imaging.Conclusion
A novel reconstruction technique based on iterative rejection of segmented k-space was developed for retrospective correction of respiratory motion in multiple average, free-breathing cine images. In comparison to standard signal averaging reconstruction, it provides higher sharpness, SNR, CNR, image quality, and rate of diagnostic quality images.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC), part of the EPSRC Doctoral Training Partnership (DTP) grant (EP/R513064/1), the British Heart Foundation (BHF) (PG/19/11/34243), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, and Siemens Healthineers. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
- Kramer CM, Barkhausen J, Bucciarelli-Ducci C, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22(17).
- Subbarao M, Choi TS, Nikzad A. Focusing techniques. SPIE Proceedings. 1992;1823.
- Vercauteren T, Pennec X, Perchant A, et al. Diffeomorphic demons: Efficient non-parametric image registration. NeuroImage. 2009;45(1):S61-72.
- McElroy S, Ferrazzi G, Nazir MS, et al. Combined simultaneous multislice bSSFP and compressed sensing for first-pass myocardial perfusion at 1.5 T with high spatial resolution and coverage. Magn Reson Med. 2020;84(6):3103-3116.
Figures
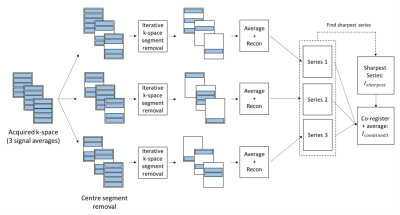
Figure 1: Summary of the proposed
reconstruction of free breathing, multiple average CINE data. The
reconstruction pipeline shown in this diagram is applied to each image (i.e.
each slice and cardiac phase) to generate a final reconstructed image Icombined3.

Figure 2: Example images reconstructed from two patients comparing the three reconstruction techniques. Image quality scores in patients #9(top row)/#14(bottom row) were 1/0 (“Standard”), 2/2 (“Sharpest)” and 2/2 (“Combined3”). Scores: 0 = non-diagnostic, 1 = diagnostic (sub-optimum image quality - presence of motion artefacts/blurring), 2 = diagnostic (excellent image quality - no motion artefacts/no blurring/sharp myocardial edges).
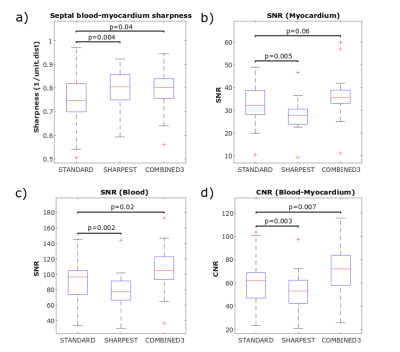
Figure
3: Quantitative analysis of sharpness, SNR and CNR over all patients for the
“Standard”, “Sharpest” and “Combined3” reconstructions: a) Septal
blood-myocardium sharpness, b) Myocardial SNR, c) LV blood pool SNR, d)
Blood/myocardium CNR. “Combined3” resulted in higher sharpness, SNR and CNR
than “Standard”.
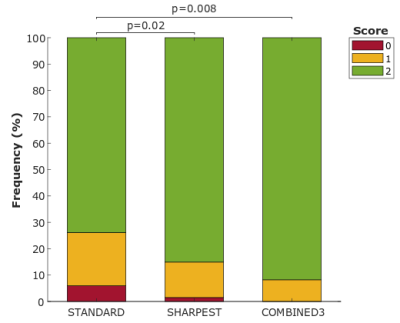
Figure 4: Qualitative image assessment.
Scores: 0 = non-diagnostic, 1 = diagnostic (sub-optimum image quality -
presence of motion artefacts/blurring), 2 = diagnostic (excellent image quality
- no motion artefacts/no blurring/sharp myocardial edges). Subjective scores for “Sharpest” and
“Combined3” were significantly higher to “Standard”.
DOI: https://doi.org/10.58530/2022/1850