1849
Feasibility of machine learning for cardiovascular function analysis in patients with repaired tetralogy of Fallot1University of Pennsylvania, Philadelphia, PA, United States, 2Children's Hospital of Philadelphia, Philadelphia, PA, United States
Synopsis
Tetralogy of Fallot (ToF) is a congenital heart disease that is typically repaired with surgery early in life, but right ventricular remodeling results in adverse events for many patients. This preliminary analysis of 8 patients investigated the feasibility of training a convolutional neural network to segment the right and left ventricles from 2-dimensional cardiovascular magnetic resonance images, resulting in Dice scores ranging from 0.73-0.91 for the left ventricular blood pool, left ventricular myocardium, and right ventricular blood pool. Machine learning shows promise to enable large-scale longitudinal studies of ToF.
Introduction
Tetralogy of Fallot (ToF) is the most common cyanotic congenital heart disease (CHD), comprising approximately 10% of CHD and 1 in 3600 births overall (1,2). Although early surgery can repair the defects associated with ToF, up to 44% of patients will go on to experience events related to pathologic right ventricular (RV) remodeling, defined as changes in RV geometry, wall thickness, and pressure-volume relationships (3). Despite significant advances in the overall survival of repaired ToF (rToF) patients, there is limited understanding of which patients will experience RV remodeling and adverse events (4). While cardiovascular magnetic resonance (CMR) imaging can be used to monitor patients, analysis of these images is user-dependent and time-consuming. Additionally, recent work has demonstrated that algorithms developed using CMR from normal hearts may not apply to the unique physiology of rToF patients, who have altered RV anatomy (5). We therefore propose to train a machine learning model to segment cardiac volumes quickly and accurately from CMR in order to identify the structural and functional parameters of rToF that are associated with RV remodeling.Methods
Patients. The Single Center Outcomes Study in ToF (SCOUT-ToF) is a longitudinal study of more than 800 rToF patients who have all received standard surgery, care, and post-repair CMR imaging at the Children’s Hospital of Philadelphia (CHOP). Follow-up imaging has been performed in >350 patients. For this preliminary feasibility study, 8 randomly-selected patients were included. Imaging was performed between 2016 and 2020.Imaging protocol. CMR studies were performed on a 1.5-T Avanto Whole Body Magnetic Resonance System (Siemens Medical Solutions, Erlangen, Germany) with a 6-channel body-array coil scanner. Imaging included steady-state free-precession cine CMR acquisitions in 4-chamber and long-axis planes and contiguous short-axis cine imaging from the atrioventricular junction through the cardiac apex. Sedation was used when appropriate according to patient’s age and ability to lie still for the scan.
Generation of training data. The left ventricular (LV) blood pool, LV myocardium, and RV blood pool were contoured from contiguous short-axis images at end-systole and end-diastole by trained experts at CHOP using cvi42 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada).
Machine learning model. A convolutional neural network based on the U-net architecture (6) was trained to segment the LV blood pool, LV myocardium, and RV blood pool from 2-dimensional short axis CMR images. Approximately 75% of scans were randomly selected as part of the training group. Training images were further augmented through rotation and scaling.
Evaluation of algorithm performance. Overlap between manual and machine learning segmentations in the test set was assessed using Dice scores (Equation 1; where X and Y are two sets of discrete data, i.e. the manual and model segmentations).
$$Eq. 1: Dice score= (2|X∩Y|)/(|X|+|Y|)$$
2-dimensional segmentations were combined with voxel sizes to calculate end-systolic volume (ESV), end-diastolic volume (EDV), and ejection fraction (EF; defined as 100%*(EDV-ESV)/EDV). ESV, EDV, and EF for each ventricle were compared between manual and model segmentations through Spearman’s rho correlation.
Results
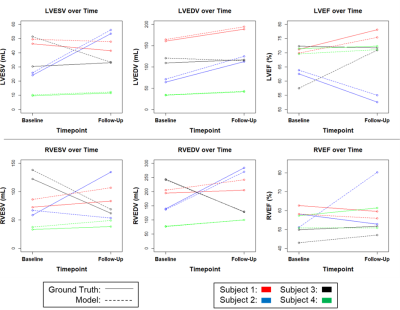
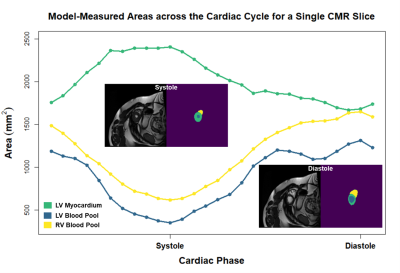
From a total of 257 available 2-dimensional CMR slices, 207 were used for training, 20 for validation, and 50 for testing. The model achieved median Dice scores of 0.91, 0.76, and 0.73 for the LV blood pool, LV myocardium, and RV blood pool, respectively. A visual comparison of ground truth and model-generated segmentations is shown in Figure 1. ESV, EDV, and EF medians and correlations for ground truth versus model-based segmentations are shown in Table 1. Spearman’s rho correlations ranged from 0.59-0.97. Additionally, four patients had CMR performed at two post-repair timepoints, with a median of 2.94 years between scans. Figure 2 shows changes in ESV, EDV, and EF for these patients. Figure 3 demonstrates model-segmented area measurements across a cardiac cycle for a single slice location in one patient.Discussion
A U-net algorithm trained on 2-dimensional CMR images from 8 patients with rToF gave modest, but promising segmentation results, with Dice scores ranging from 0.73-0.91 and Spearman’s rho correlations of 0.59-0.97. Given its success with this small dataset, we anticipate that training with a larger proportion of the SCOUT-ToF cohort will greatly improve performance. To increase available training data for this study, we chose to use 2-dimensional slices rather than 3-dimensional volumes. However, the algorithm appeared to struggle at more posterior as well as more apical slices where the heart can be difficult to distinguish from background anatomy. Training the model on 3-dimensional cardiac volumes in the future may help mitigate this issue by providing additional spatial context through contiguous image slices, and training with cine images will provide relative time-scale information. Although we lacked patient demographics, all our contours were generated by trained clinicians and technicians, making our results representative of those generated in the clinic, and future studies will include this patient-level data.Conclusion
The preliminary outcomes of this study demonstrate the potential of machine learning to accurately segment the LV and RV from CMR images in patients with rToF morphology. Future studies will include CMR images from additional patients and sites as well as 3-dimensional training data, and will evaluate several model architectures to optimize speed and accuracy. The results of these efforts will enable large-scale longitudinal studies of RV remodeling in rToF.Acknowledgements
No acknowledgement found.References
1. Villafane J, Feinstein JA, Jenkins KJ, Vincent RN, Walsh EP, Dubin AM, Geva T, Towbin JA, Cohen MS, Fraser C, Dearani J, Rosenthal D, Kaufman B, Graham TP, Jr., Adult C, Pediatric Cardiology Section ACoC. Hot topics in tetralogy of Fallot. J Am Coll Cardiol 2013;62(23):2155-2166.
2. Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet 2009;374(9699):1462-1471.
3. Kret M, Arora R. Pathophysiological basis of right ventricular remodeling. J Cardiovasc Pharmacol Ther 2007;12(1):5-14.
4. van der Ven JPG, van den Bosch E, Bogers A, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000Res 2019;8.
5. Tandon A, Mohan N, Jensen C, Burkhardt BEU, Gooty V, Castellanos DA, McKenzie PL, Zahr RA, Bhattaru A, Abdulkarim M, Amir-Khalili A, Sojoudi A, Rodriguez SM, Dillenbeck J, Greil GF, Hussain T. Retraining Convolutional Neural Networks for Specialized Cardiovascular Imaging Tasks: Lessons from Tetralogy of Fallot. Pediatr Cardiol 2021;42(3):578-589.
6. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. 2015. Springer. p 234-241.
Figures