1831
Evidence for an intercollicular auditory push/pull mechanism revealed by BOLD fMRI1Champalimaud Research, Champalimaud Centre for the Unknown, Lisboa, Portugal
Synopsis
The role of the rodent inferior colliculus (IC) in binaural integration is of great interest especially for sound localization and processing of interaural level difference (ILD). Yet, many IC underlying mechanisms remain unclear. Here, we find evidence for a push/pull mechanism in IC, with contralateral (positive BOLD) activity exerting dominance over ipsilateral (negative BOLD) activity, possibly as means of a sound localization/lateralization.
Introduction
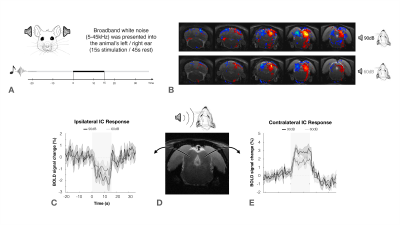
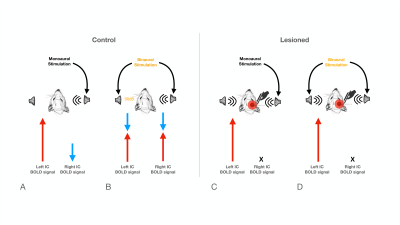
The inferior colliculus (IC) plays an important role in binaural integration in rodents, specifically for sound localization. However, the specific processing mechanisms remain mostly unclear. Evidence suggests that ICs do not operate in isolation; the largest afferent source to each IC has been suggested to be their contralateral IC, through excitation, inhibition or a combination of both1-3. Furthermore, it has been suggested that contralateral neurons exert dominance by a “push-pull”-like mechanism4, with contralaterally dominant excitation and more bilaterally balanced inhibition, from an ipsilaterally mediated scaling of contralateral response4. Moreover, intrinsic optical imaging responses upon bilateral stimulation were shown to be weaker than those arising upon contralateral stimulation, suggesting an ipsilateral neural pathway suppressing neural responses is evoked contralaterally5-6. Previous experiments demonstrated that monoaural auditory stimulation induces both positive contralateral7-9 and negative ipsilateral10 BOLD responses under specific conditions (Fig.1). Monaural auditory stimulation evokes a positive contralateral response and a negative ipislateral one; here, we hypothesized that similarly, binaural BOLD responses in each IC would not only be comprised of the contralateral positive response, but its summation with a negative ipsilateral influence. Further, we disrupt the mechanism by unilaterally lesioning IC (Fig.2c).Methods
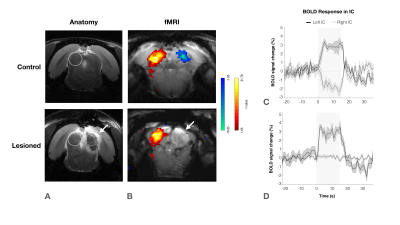
Animal experiments were preapproved by the institutional and national authorities and carried out according to European Directive 2010/63. Adult Long Evans rats (total n=11) were sedated with medetomidine11, while temperature and respiration rates were monitored and kept stable. Animals were divided into two groups, “Control” (n=6) and “Lesioned” (n=5) (Fig.2). Controls consist of healthy animals, while Lesioned animals were previously subjected to the injection of Ibotenic Acid in the rightside IC.MRI experiments: A 9.4T BioSpec scanner (Bruker, Karlsruhe, Germany) with an 86mm quadrature resonator for transmittance and a 4-element array cryoprobe (Bruker, Fallanden, Switzerland) for signal reception was used. Data was acquired under medical air (28%O2) with a GE-EPI sequence (TE/TR=14/1000ms, FOV=20x13mm2, in-plane resolution=250x250μm2, slice thickness=1mm, tacq=6min45s)
Auditory Stimulation: Broadband (5-45kHz) white noise at 90/60dB was presented into the animal’s ears using a specialized setup. The stimulation paradigm consisted of six repetitions of 15s stimulation and 45s rest (Fig.1a).
IC Lesions: Anatomical scans of each individual animal were acquired and superimposed on a rat brain atlas12, where injection coordinates were determined. Lesions were performed through a craniotomy by injecting ibotenic acid into the right IC. Animals were scanned 20 to 30 hours after surgery to prevent/minimize neuronal reorganization.
Data analysis: fMRI pre-processing steps included manual outlier correction; slice-timing (sinc-interpolation); smoothing (FWHM=0.250mm isotropic), followed by GLM and ROI analysis.
Results
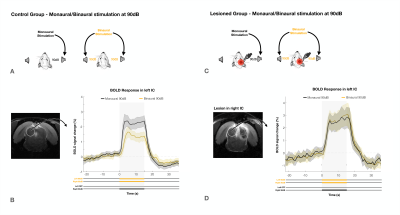
The effectiveness of lesion induction was verified post-surgery, both through anatomical scans (location and extent of lesions), and functional acquisitions (lack of activation in the lesioned IC) (Fig.2b).With monaural (black), and binaural (yellow) stimulation at 90dB for the control group (Fig.3a), a decrease in BOLD maximum amplitude from monaural to binaural stimulation was observed (Fig.3b). Interestingly, in the lesioned group (Fig3c) no discernible differences could be found between monaural and binaural stimulations (Fig3d).
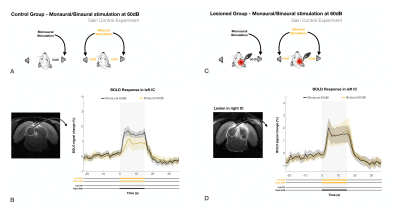
To account for possible gain control effects, the experiment was repeated at lower intensity of 60dB (Fig.4). In the control group (Fig.4a) a decrease in BOLD signal from monaural to binaural stimulation was again observed, (Fig.4b), while no discernible differences were found for the lesioned group (Fig.4d). In both groups, the overall response amplitudes were lower, as expected with lower intensity stimuli.
Discussion
We have shown that binaural stimulation produces lower BOLD responses when compared to monaural stimulation at the same intensity (Fig.3a/4a), and that unilaterally lesioning IC abolishes these differences (Fig.3b/4b). This suggests the existence of a strong auditory push/pull BOLD mechanism during intercollicular communication.In the monaural regime there is no competition for auditory processing, as only one sound source is present. On the other hand, we show the result of a binaural stimulation is not only each individual positive contralateral response but rather the summation of both positive and negative responses (Fig.5). This competition drives down each colliculli’s BOLD responses, which indicates that the mechanism we see generating negative BOLD with monaural stimulation is still acting during binaural stimulation. This is further confirmed by its disruption upon unilaterally lesioning IC.
The loud amplitudes of the first experiment (90dB) could be close to a saturation point for IC processing13-14. Thus, the lower BOLD responses with binaural competition could simply be a manner of regulating gain between the two IC, as previously suggested1,15,16, with stimulation of the commissure of the IC producing both excitatory/inhibitory effects on IC neurons17–19. However, we were able to deconfound this effect using the lower volume experiment (Fig.4), (60dB) which yielded very similar results, thereby excluding gain control as a dominant factor in this phenomenon.
Conclusion
We present clear evidence for an auditory push/pull mechanism revealed through the BOLD response, with contralateral (positive) activity exerting dominance over ipsilateral (negative) responses, possibly as means of a ILD comparison mechanism (Fig.5). This effect is preserved going from monaural to binaural stimulation, regardless of the presented amplitude (90/60dB), which could negate the hypothesis of it being purely a gain regulation mechanism and further implicate its role in sound lateralization/localization.Acknowledgements
No acknowledgement found.References
1. Saldaña E, Merchań MA. Intrinsic and commissural connections of the rat inferior colliculus. J. Comp. Neurol. 1992 doi: 10.1002/cne.903190308.
2. Kuwabara N, Zook JM. Geniculo-collicular descending projections in the gerbil. Brain Res. 2000 doi: 10.1016/S0006-8993(00)02695-0.
3. Caicedo A, Herbert H. Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J. Comp. Neurol. 1993 doi: 10.1002/cne.903280305.
4. Xiong XR, Liang F, Li H, et al. Interaural level difference-dependent gain control and synaptic scaling underlying binaural computation. Neuron 2013 doi: 10.1016/j.neuron.2013.06.012.
5. Tsytsarev V, Tanaka S. Intrinsic optical signals from rat primary auditory cortex in response to sound stimuli presented to contralateral, ipsilateral and bilateral ears. Neuroreport 2002 doi: 10.1097/00001756-200209160-00019.
6. Nelken I, Bizley JK, Nodal FR, Ahmed B, King AJ, Schnupp JWH. Responses of auditory cortex to complex stimuli: Functional organization revealed using intrinsic optical signals. J. Neurophysiol. 2008 doi: 10.1152/jn.00469.2007.
7. Cheung MM, Lau C, Zhou IY, et al. BOLD fMRI investigation of the rat auditory pathway and tonotopic organization. Neuroimage 2012 doi: 10.1016/j.neuroimage.2012.01.087.
8. Zhang JW, Lau C, Cheng JS, et al. Functional magnetic resonance imaging of sound pressure level encoding in the rat central auditory system. Neuroimage 2013 doi: 10.1016/j.neuroimage.2012.09.069.
9. Lau C, Zhang JW, Cheng JS, Zhou IY, Cheung MM, Wu EX. Noninvasive fMRI Investigation of Interaural Level Difference Processing in the Rat Auditory Subcortex. PLoS One 2013 doi: 10.1371/journal.pone.0070706.
10. Frederico Severo, Rita Gil NS. Monoaural auditory stimulation induces negative BOLD along the rat auditory pathway. In: 30th ISMRM & SMRT Annual Meeting & Exhibition. ; 2021.
11. Weber R, Ramos-Cabrer P, Wiedermann D, Van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage 2006 doi: 10.1016/j.neuroimage.2005.08.028.
12. Paxinos G, Charles Watson. The Rat Brain in Stereotaxic Coordinates Sixth Edition.; 2007.
13. Manohar S, Spoth J, Radziwon K, Auerbach BD, Salvi R. Noise-induced hearing loss induces loudness intolerance in a rat Active Sound Avoidance Paradigm (ASAP). Hear. Res. 2017;353:197–203 doi: 10.1016/j.heares.2017.07.001.
14. N.Flores E, Liberman C, Duggan A, et al. A Non-canonical Pathway from Cochlea to Brain Signals Tissue-Damaging Noise. Curr. Biol. 2015;25:606–612.
15. Adams JC. Crossed and descending projections to the inferior colliculus. Neurosci. Lett. 1980 doi: 10.1016/0304-3940(80)90246-3.
16. Ayala YA, Malmierca Dr. MS. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front. Neural Circuits 2012 doi: 10.3389/fncir.2012.00089.
17. Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. J. Neurosci. 2009 doi: 10.1523/JNEUROSCI.3454-09.2009.
18. Moore DR, Kotak VC, Sanes DH. Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J. Neurophysiol. 1998 doi: 10.1152/jn.1998.80.5.2229.
19. Reetz G, Ehret G. Inputs from three brainstem sources to identified neurons of the mouse inferior colliculus slice. Brain Res. 1999 doi: 10.1016/S0006-8993(98)01230-X.
Figures