1828
fMRI in the Mouse Spinal Cord during peripheral somatosensory Stimulation
Amon Allelein1, Bruno Pradier1, Huifen Chen2, and Cornelius Faber2
1Radiology, UKM, Muenster, Germany, 2UKM, Muenster, Germany
1Radiology, UKM, Muenster, Germany, 2UKM, Muenster, Germany
Synopsis
The spinal cord is an important hub for the integration and processing of peripheral somatosensory stimuli within the central nervous system, however, mouse spinal cord functional imaging is not yet established. We successfully performed BOLD fMRI in the mouse spinal cord using 1 electric and 3 different mechanical stimulation modalities in one session. Graph theory-based network analyses showed strong positive correlations within the dorsal spinal cord laminae of L4 and L5. A negative correlation between the dorsal and ventral regions within the same segment suggests the presence of a vascular steal effect.
INTRODUCTION
The spinal cord is an important hub for the integration and processing of peripheral somatosensory stimuli within the central nervous system. With the continuous development and improvement of fMRI studies of the brain it is possible to show the effects of different stimulation modalities in mice. However, mouse spinal cord functional imaging is not yet routinely established because of many technical difficulties, including the small tissue volume and resulting low resolution, or the large amount of movement artifacts and distortions. Here, we report functional imaging of mouse spinal cord using 3 different mechanical and 1 electrical modality applied to the hind paw.
METHODS
Anesthesia Regimen was employed as recently described1 (for animal preparation: 1.5 – 2% ISO ; for stimulation: combined anesthesia with 0.2% isoflurane and 0.2mg/kg*h medetomidine).
MRI equipment and sequence: Measurements were conducted on a 9.4 T Bruker Biospec 94/20 small animal scanner (Bruker Biospin GmbH, Ettlingen, Germany) using a 1 cm surface coil positioned under the last rib. The mice were positioned in supine position on an animal cradle to allow free breathing and to reduce motion artifacts. Anatomical images of the spinal cord were acquired (FLASH, TR/TE 350/5.4 ms) (Fig. 1A). BOLD fMRI measurements were performed for 10 minutes using a spin echo planar imaging sequence (SE-EPI, TR=1000 ms, TE=40 ms, 8 slices, slice thickness 1 mm, resolution 140 ´ 140 µm2, 250 x 250 matrix) with the 4th slice lying above the L4 area of the lumbar spinal cord.
Mechanical stimulation: an in-house built rotating pneumatic mechanical stimulator was used to deliver 3 different mechanical modalities: cotton swab (non-noxious, activating peripheral Aβ fibers), von Frey filament (noxious/non-noxious, activating Aβ and Aδ fibers), and pinprick (noxious, predominantly activating Aδ fibers).
Stimulation design: For mechanical hind paw stimulations a block design was used with 10s OFF – 10s ON – 10s OFF and a total of 30 repetitions. The mechanical stimulation was performed at 1Hz to prevent wind-up. For the electrical stimulation a block design of 10s OFF – 5s ON – 15s OFF was used. The stimulation was performed at 2 mA with 9 Hz and a pulse length of 2 ms (Fig.1B).
Analysis: The SE-EPI scans were preprocessed as previously puublished1. Following slice timing and motion correction and smoothing, the FIR analysis was performed using SPM. Further graph theory-based network analyses were performed using MagnAN (BioCom, Uttenreuth).
RESULTS
We successfully performed BOLD fMRI in the mouse spinal cord using 1 electric and 3 different mechanical stimulation modalities in one session. We performed a FIR-based GLM analysis of BOLD activation in the spinal cord during hind paw stimulation (Fig. 2A), which was strongest in dorso-medial part of L4/L5 (Fig. 2B). Next, we find that electrical stimulation leads to a preferential activation of the dorso-medial laminae of the spinal cord whereas the ventral spinal cord of L4/L5 shows little-to-no activation (Fig. 3). Moreover, we find an intensity-dependent increase of activated voxels comparing CS- with vF stimulation. Interestingly, we could not detect differences in activation of ipsi- or contralateral hemispheres. Using the spinal cord atlas with 24 regions, we performed a cross-correlation analysis for the lumbar segments L3 – L6 (Fig. 4A). Remarkably, the network analysis shows strong positive correlation between the dorsal regions of L4 and L5 across all stimulation modalities. This effect is strongest during pinprick and electrical stimulations. Further, we found positive correlation between the ventral regions of L4 and L5 for all stimulation modalities. Interestingly, we observe a negative correlation in L4 and L5 between ventral and dorsal laminae (Fig. 4B). This effect was strongest with pinprick stimulation, but also present in electrical, von Frey and cotton swab stimulation. Consistent with the FIR analysis we could not detect differences between ipsi- and contralateral sides.
DISCUSSION
The different numbers of activated voxels observed during mechanical stimulation suggests peripheral input- (and thereby modality)-dependent processing in the dorsal spinal cord: non-noxious stimulation (CS) leads to a reduced activation compared to noxious stimulation (PP). The positive correlation in spinal dorsal regions suggests synchronous activity across different lumbar segments where the afferent sensory information is processed. These changes were strongest in the dorsal regions of L4 and L5. Further, the positive correlation in the ventral regions of L4 and L5 suggest an activation of motoneurons in the reflex arch following stimulation. The negative correlation between the dorsal and ventral regions of L4 and L5 might be explained by the vascular-steal-effect: the blood flow is redirected from ventral to dorsal regions to meet the increased demand for oxygen of the dorsal horn during stimulation.
CONCLUSION
It is possible to perform fMRI in the mouse spinal cord. Hind paw stimulation results in increased BOLD activation of dorsomedial spinal cord, which is negatively correlated to activation in the ventral part, thereby giving rare insights into hemodynamics of the spinal cord signal processing.
The spinal cord is an important hub for the integration and processing of peripheral somatosensory stimuli within the central nervous system. With the continuous development and improvement of fMRI studies of the brain it is possible to show the effects of different stimulation modalities in mice. However, mouse spinal cord functional imaging is not yet routinely established because of many technical difficulties, including the small tissue volume and resulting low resolution, or the large amount of movement artifacts and distortions. Here, we report functional imaging of mouse spinal cord using 3 different mechanical and 1 electrical modality applied to the hind paw.
METHODS
Anesthesia Regimen was employed as recently described1 (for animal preparation: 1.5 – 2% ISO ; for stimulation: combined anesthesia with 0.2% isoflurane and 0.2mg/kg*h medetomidine).
MRI equipment and sequence: Measurements were conducted on a 9.4 T Bruker Biospec 94/20 small animal scanner (Bruker Biospin GmbH, Ettlingen, Germany) using a 1 cm surface coil positioned under the last rib. The mice were positioned in supine position on an animal cradle to allow free breathing and to reduce motion artifacts. Anatomical images of the spinal cord were acquired (FLASH, TR/TE 350/5.4 ms) (Fig. 1A). BOLD fMRI measurements were performed for 10 minutes using a spin echo planar imaging sequence (SE-EPI, TR=1000 ms, TE=40 ms, 8 slices, slice thickness 1 mm, resolution 140 ´ 140 µm2, 250 x 250 matrix) with the 4th slice lying above the L4 area of the lumbar spinal cord.
Mechanical stimulation: an in-house built rotating pneumatic mechanical stimulator was used to deliver 3 different mechanical modalities: cotton swab (non-noxious, activating peripheral Aβ fibers), von Frey filament (noxious/non-noxious, activating Aβ and Aδ fibers), and pinprick (noxious, predominantly activating Aδ fibers).
Stimulation design: For mechanical hind paw stimulations a block design was used with 10s OFF – 10s ON – 10s OFF and a total of 30 repetitions. The mechanical stimulation was performed at 1Hz to prevent wind-up. For the electrical stimulation a block design of 10s OFF – 5s ON – 15s OFF was used. The stimulation was performed at 2 mA with 9 Hz and a pulse length of 2 ms (Fig.1B).
Analysis: The SE-EPI scans were preprocessed as previously puublished1. Following slice timing and motion correction and smoothing, the FIR analysis was performed using SPM. Further graph theory-based network analyses were performed using MagnAN (BioCom, Uttenreuth).
RESULTS
We successfully performed BOLD fMRI in the mouse spinal cord using 1 electric and 3 different mechanical stimulation modalities in one session. We performed a FIR-based GLM analysis of BOLD activation in the spinal cord during hind paw stimulation (Fig. 2A), which was strongest in dorso-medial part of L4/L5 (Fig. 2B). Next, we find that electrical stimulation leads to a preferential activation of the dorso-medial laminae of the spinal cord whereas the ventral spinal cord of L4/L5 shows little-to-no activation (Fig. 3). Moreover, we find an intensity-dependent increase of activated voxels comparing CS- with vF stimulation. Interestingly, we could not detect differences in activation of ipsi- or contralateral hemispheres. Using the spinal cord atlas with 24 regions, we performed a cross-correlation analysis for the lumbar segments L3 – L6 (Fig. 4A). Remarkably, the network analysis shows strong positive correlation between the dorsal regions of L4 and L5 across all stimulation modalities. This effect is strongest during pinprick and electrical stimulations. Further, we found positive correlation between the ventral regions of L4 and L5 for all stimulation modalities. Interestingly, we observe a negative correlation in L4 and L5 between ventral and dorsal laminae (Fig. 4B). This effect was strongest with pinprick stimulation, but also present in electrical, von Frey and cotton swab stimulation. Consistent with the FIR analysis we could not detect differences between ipsi- and contralateral sides.
DISCUSSION
The different numbers of activated voxels observed during mechanical stimulation suggests peripheral input- (and thereby modality)-dependent processing in the dorsal spinal cord: non-noxious stimulation (CS) leads to a reduced activation compared to noxious stimulation (PP). The positive correlation in spinal dorsal regions suggests synchronous activity across different lumbar segments where the afferent sensory information is processed. These changes were strongest in the dorsal regions of L4 and L5. Further, the positive correlation in the ventral regions of L4 and L5 suggest an activation of motoneurons in the reflex arch following stimulation. The negative correlation between the dorsal and ventral regions of L4 and L5 might be explained by the vascular-steal-effect: the blood flow is redirected from ventral to dorsal regions to meet the increased demand for oxygen of the dorsal horn during stimulation.
CONCLUSION
It is possible to perform fMRI in the mouse spinal cord. Hind paw stimulation results in increased BOLD activation of dorsomedial spinal cord, which is negatively correlated to activation in the ventral part, thereby giving rare insights into hemodynamics of the spinal cord signal processing.
Acknowledgements
No acknowledgement found.References
1. Pradier B, Wachsmuth L, Nagelmann N, et al. Combined resting state-fMRI and calcium recordings show stable brain states for task-induced fMRI in mice under combined ISO/MED anesthesia. Neuroimage. Published online October 9, 2021:118626. doi:10.1016/J.NEUROIMAGE.2021.118626Figures
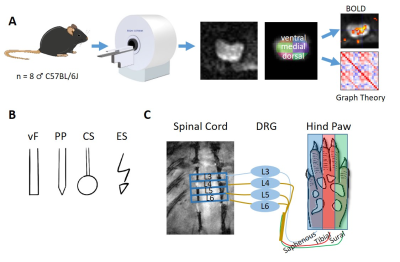
Fig.1A)
Workflow for fMRI in the spinal cord. SE-EPI fMRI scans were segmented using an
in-house developed spinal cord atlas with 24 regions: 4 segments (lumbar
segment 3-6), each consisting of 6 subdivisions (left and right dorsal, medial
and ventral aspects). B) 4 different stimulation modalities were
applied to the central aspect of the hind paw: electrical stimulation (ES),
cotton swab (CS), von Frey filaments (vF) and pinprick (PP). C)
Stimulation is expected to project through the tibial division of the sciatic
nerve to lumbar segments of the spinal cord.
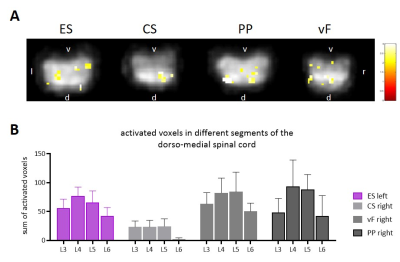
Fig. 2A) BOLD-map of activated voxels
overlaid onto smoothed SE-EPI images of the spinal cord following stimulation
with 4 different modalities. B) Sum of activated voxels in the dorso-medial
spinal cord of different lumbar segments (L3-L6) Maximal activation can be
found in segments L4 and L5.
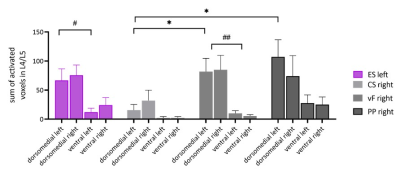
Fig 3) Sum of activated voxels in
the dorsomedial and ventral spinal cord of L4 and L5 following different
stimulation modalities applied to the hind paw. All stimulation modalities
result in a preferential activation of the dorsomedial laminae of the spinal
cord, whereas the ventral aspect of the spinal cord showed little activation.
Electrical stimulation (ES), applied to the left hind paw. Mechanical
stimulations included cotton swab (CS), von Frey filaments (vF) and
pinprick (PP) were applied to the right hind paw.
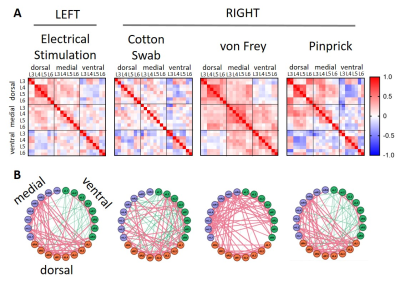
Fig.4) Graph theory-based network
analysis of spinal cord BOLD time courses during peripheral stimulation of the
hind paw. A) dorsal and ventral structure of L4 and L5 showed strong clustering
across all modalities. Interestingly, BOLD time courses were negatively
correlated between dorsal and ventral structures. B) Circular representation of
network activity shows predominantly positive correlations in dorso-medial
structures and negative correlations in ventral structures.
DOI: https://doi.org/10.58530/2022/1828