1822
Resolving activity suppression in the rat visual pathway using fMRI combined with lesions1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon PT, Lisbon, Portugal
Synopsis
Monocular visual stimulation with short inter-stimulus intervals (ISIs) evokes (i) negative BOLD responses (NBRs) in the contralateral superior colliculus (cSC), and (ii) positive BOLD responses (PBRs) in the ipsilateral superior colliculus (iSC). This pattern suggests a potential "push-pull" mechanism between SCs possibly evoked by (mostly inhibitory) tectotectal projections. Here, we mapped activity in the entire visual pathway using fMRI, and modulated cSC inputs through lesions in visual cortex and SC to dissect collicular communication mechanisms. While, cortical context potentiated a putative “push-pull” mechanism, further silencing of iSC resulted in PBRs in cSC, suggesting that iSC exerts suppression on cSC.
Introduction
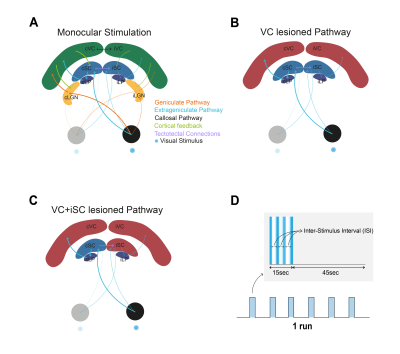
Superior colliculus (SC) is an important structure for multimodal integration1,2. While deeper layers are involved in saccade control, superficial layers receive direct inputs from the retina and respond strongly to looming stimuli and light flashes1,2. Within the visual pathway, SC receives inputs from several other structures (Fig.1A): corticotectal feedback from visual cortex (VC) mainly consisting of nonGABAergic projections acting as gain control of SC responses1-4, and tectotectal projections connecting both SCs and exerting an overall inhibitory effect between SCs4-6. fMRI allows the interrogation of activity in the entire visual pathway. Negative BOLD responses (NBRs), mainly thought to represent activity suppression, were measured in the contralateral SC (cSC) and visual cortex (VC) at short inter-stimulus intervals (ISIs) upon monocular stimulation. Here, we aim to understand the impact of tectotectal and cortical inputs in inter-collicular communication using BOLD fMRI (Fig.1B-C).Methods
Animal experiments were preapproved by institutional and national authorities and were carried out according to European Directive 2010/63.Adult Long Evans rats were sedated with medetomidine while monitoring temperature and respiration rate.
MRI: A 9.4T BioSpec scanner (Bruker, Germany) with an 86mm quadrature resonator for transmittance and a 4-element array cryoprobe7,8 (Bruker, Switzerland) for signal reception was used. fMRI was acquired at 95%O2 with a SE-EPI sequence (TE/TR=40/1500ms, FOV=18x16.1mm2, resolution=268x268μm2, slice thickness=1.5mm, tacq=6min45s).
Ibotenic Acid Lesions: Injections of 1% ibotenic9 solution diluted in a 0.1M NaOH solution were performed along the left SC and/or both VCs. Animals were imaged 1 week after surgery to avoid inflammation.
Stimulation: A blue LED (𝜆=470nm) was used for monocular stimulation with ISIs of 990, 56 and 30ms. The stimulation paradigm is shown in Fig.1D.
Data analysis: fMRI pre-processing included manual outlier correction; slice-timing correction; smoothing (3D Gaussian kernel, FWHM=0.268mm isotropic); mean volume realignment and co-registration to a T2-weighted anatomical reference. An HRF peaking at 1s was convolved with the paradigm. A minimum significance level of 0.001 (FDR corrected) with a minimum cluster size of 20 voxels were used.
Time-courses were calculated by manually drawing atlas-based10 ROIs and detrending individual runs with a 5th-order polynomial fit to the resting periods.
For statistical comparison the Kruskal-Wallis test was used as data rejected the Shapiro-Wilk11 test’s null hypothesis.
Results
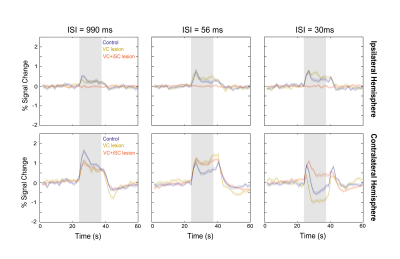
Upon monocular visual stimulation of normal controls, BOLD t-maps (Fig.2A) show the appearance of cortical and cSC NBRs at short-ISIs, along with small ipsilateral SC (iSC) positive BOLD responses (PBRs). Particularly for cSC, time-courses reveal two-peak shaped NBRs (Fig.2A, black arrows).Lesioning VC (Fig.1B and 2B) potentiated iSC PBRs and cSC NBRs, with the latter retaining the two-peak profile. Further isolation of cSC from iSC tectotectal inputs (Fig.1C and 2C) resulted in cSC PBRs. Interestingly cSC PBRs retain the two-peak profile characteristic of short-ISI SC responses.
We then overlapped short-ISI SC responses for the control, VC-lesioned and VC+iSC-lesioned regimes (Fig.3). For the longest ISI, the control and lesioned regimes exhibit similar response profiles with the contralateral lesion conditions responses evidencing reduced BOLD amplitude. As the ISI decreases, while ipsilateral responses of VC-lesioned regime exhibit a small amplitude increase compared to controls, the contralateral responses show an amplitude increase for intermediate ISI but stronger negative amplitude for the shortest ISI. After lesioning iSC, contralateral responses remained similar to VC-lesioned regime for longer ISIs, but for the shortest ISI, PBRs appeared.
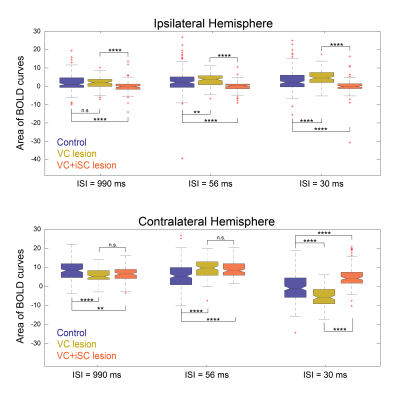
Statistical comparison of the different BOLD curve areas (Fig.4) confirmed the significant difference between control iSC and VC-iSC-lesioned curves for all ISIs. Furthermore, control cSC BOLD curve areas were found to be significantly different from lesioned regimes for all ISIs, but only at the shortest ISI a significant difference between the two lesioned regimes was detected.
Discussion
Our results suggest that inter-collicular communication involves a “push-pull” mechanism. In control conditions short ISIs are associated with strong NBRs in cSC, likely due to response adaptation when the necessary period for excitability recovery is not reached12. At the same time, increased PBRs are triggered in the iSC. This positive/negative BOLD interaction between the colliculi could be evoked by (besides the retina) (1) cortical feedback, thought to have a gain control modulatory effect13-16; and/or (2) tectotectal connections, responsible for the reciprocal inhibition between SCs4,6, (although excitatory connections may also be established5, mostly in middle/deep layers of SC). When cortical feedback is silenced, the amplification of PBRs/NBRs in control conditions for iSC/cSC is in agreement with VC’s modulatory role. Moreover, iSC PBRs and cSC NBRs observed following cortical lesioning suggest a prominent role of tectotectal connections in shaping SCs responses (at least in the short-ISI regime). If we hypothesize that cSC NBRs at short ISIs correspond to neuronal suppression, then the strong upward regulation of such responses upon silencing iSC might indicate that iSC actively exerts a suppression effect in cSC during response adaptation at short ISIs.We also found a strong and persistent two-peaked profile characteristic of SC BOLD responses at short-ISI regime. We posit that these peaks are likely related with onset/offset signals16,17-19. This itself is an interesting finding that may require attention when analysing BOLD fMRI data.
Conclusions
A putative “push-pull” mechanism between the superior colliculi was observed and modulated by lesions. Our results suggest that tectotectal connections play an important role in visual processing.Acknowledgements
The authors would like to thank Renata Cruz and Beatriz Cardoso for the brain extractions in order to perform post-hoc histological confirmation of lesion localisation.
The authors would also like to thank Francisca Fernandes for the help in fMRI data analysis.
References
[1] Zhao, Xinyu, Mingna Liu, and Jianhua Cang. "Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice." Neuron 84.1 (2014): 202-213.
[2] Cang, Jianhua, et al. "Visual function, organization, and development of the mouse superior colliculus." Annual review of vision science 4 (2018): 239-262.
[3] Bereshpolova, Yulia, et al. "The impact of a corticotectal impulse on the awake superior colliculus." Journal of Neuroscience 26.8 (2006): 2250-2259.
[4] Goodale, M. A. "Cortico-tectal and intertectal modulation of visual responses in the rat's superior colliculus." Experimental Brain Research 17.1 (1973): 75-86.
[5] Olivier, Etienne, et al. "Evidence for glutamatergic tectotectal neurons in the cat superior colliculus: a comparison with GABAergic tectotectal neurons." European Journal of Neuroscience 12.7 (2000): 2354-2366.
[6] Appell, Peter P., and Mary Behan. "Sources of subcortical GABAergic projections to the superior colliculus in the cat." Journal of Comparative Neurology 302.1 (1990): 143-158.
[7] Niendorf, Thoralf, et al. "Advancing cardiovascular, neurovascular and renal magnetic resonance imaging in small rodents using cryogenic radiofrequency coil technology." Frontiers in pharmacology 6 (2015): 255.
[8] Baltes, Christof, et al. "Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe." NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo22.8 (2009): 834-842.
[9] Pai, Shraddha S., et al. "Minimal impairment in a rat model of duration discrimination following excitotoxic lesions of primary auditory and prefrontal cortices." Frontiers in systems neuroscience 5 (2011): 74.
[10] Paxinos, George, and Charles Watson. The rat brain in stereotaxic coordinates: hard cover edition. Elsevier, 2006.
[11] Shapiro, Samuel Sanford, and Martin B. Wilk. "An analysis of variance test for normality (complete samples)." Biometrika52.3/4 (1965): 591-611;
[12] Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol. 1997;504 ( Pt 3)(Pt 3):629-639. doi:10.1111/j.1469-7793.1997.629bd.x.
[13] De Franceschi, Gioia, and Samuel G. Solomon. "Visual response properties of neurons in the superficial layers of the superior colliculus of awake mouse." The Journal of physiology 596.24 (2018): 6307-6332.
[14] Zhao, Xinyu, Mingna Liu, and Jianhua Cang. "Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice." Neuron 84.1 (2014): 202-213.
[15] Liang, Feixue, et al. "Sensory cortical control of a visually induced arrest behavior via corticotectal projections." Neuron86.3 (2015): 755-767.
[16] Cang, Jianhua, et al. "Visual function, organization, and development of the mouse superior colliculus." Annual review of vision science 4 (2018): 239-262.
[17] Seabrook, Tania A., et al. "Architecture, function, and assembly of the mouse visual system." Annual review of neuroscience 40 (2017): 499-538.
[18] De Franceschi, Gioia, and Samuel G. Solomon. "Visual response properties of neurons in the superficial layers of the superior colliculus of awake mouse." The Journal of physiology 596.24 (2018): 6307-6332.
[19] Bytautiene, Juntaute, and Gytis Baranauskas. "Rat superior colliculus neurons respond to large visual stimuli flashed outside the classical receptive field." PloS one 12.4 (2017): e0174409.
Figures