1820
Evaluation of dynamic 2D-EPI acquisitions for fetal brain tracking with neural networks1Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2King's College London, London, United Kingdom
Synopsis
Fetal MRI and MRS are often compromised due to unpredictable fetal motion and commonly require multiple repetitions for diagnostic studies. To overcome these limitations, we are working towards developing a deep-learning based automatic MRI fetal motion tracking method. Our method uses, as input, rapid dynamic multi-echo 2D-EPI acquisitions and is based on a 3D U-Net for brain localisation and translational motion parameters estimation for brain tracking. The results show rapid low-resolution acquisitions contain sufficient information to allow automated fetal brain localisation. Our technique can be used to assess fetal movements and to build navigation systems for fetal MRI/MRS.
Introduction
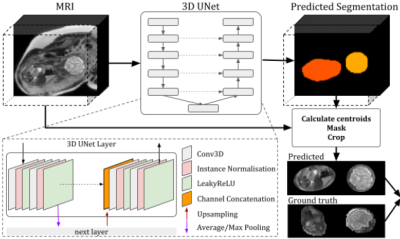
Fetal brain MRI offers unique insights into life before birth. Fetal MR spectroscopy can also probe metabolic processes involved in human development non-invasively. One of the main challenges of fetal MR is, however, the unpredictable fetal motion. In MR imaging this is currently addressed with rapid single-shot acquisitions, followed by post processing such as SVR [1]. For motion robustness, fetal MRS would benefit from brain tracking similar to its implementation in adults [2]; this uses 3D-EPI volumetric navigator images processed with scan-to-scan position estimation algorithms developed for prospective motion corrected fMRI [3]. Its extension to fetal MRS is, however, challenging due to the surrounding uterine environment and maternal tissue. While examples of fetal 3D-EPI navigators have been shown [4][5], they are prone to motion artefact, and their processing involved manual intervention and slow conventional registration using FSL-FLIRT [6]. In this study we evaluate the performance of an automated 3D-UNet [7] to estimate fetal head position, exploring network performance on data with different contrasts acquired with dynamic multi-echo 2D-EPI as a potential real-time brain tracking protocol.Methods
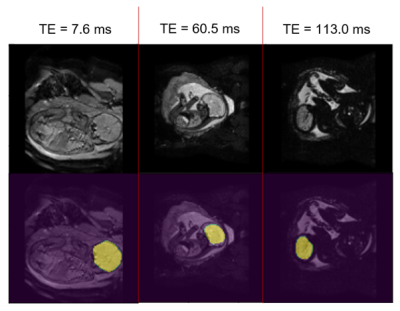
The method was evaluated on 24 fetal scans obtained at 1.5T with a multi-echo Gradient Echo EPI sequence, acquired as part of the CARP (cardiac and placenta imaging), study TR=6.4 s, voxel size=1.6x1.6x2.5 mm3, with 30 dynamic time points and 3 echo-times (TE1/TE2/TE3=7.9/60.5/113.0 ms). 1- Fetal brain localisation: A 3D U-Net originally developed for high-resolution, SVR-processed [1] T2-weighted structural images [7] was used to generate brain masks in all consecutive time points, allowing the removal of fetal and maternal tissue that can confound fetal brain tracking. The network was trained on images from the 3 echo-times combined for 6 fetal dynamic datasets (GA 24-37 weeks). Ground truth segmentations for the training were obtained by manually segmenting the brain on the first time point of each dynamic dataset, and transferring this segmentation to all successive time points applying automated label propagation using the localisation network. The trained model was run on the three echo-times images of 12 unseen dynamic datasets (GA 27-37 weeks). 2- Fetal brain tracking: Fetal head location was then estimated by extracting the centre of mass coordinates of brain segmentations at each consecutive time point; fetal head translation parameters were plotted.Results
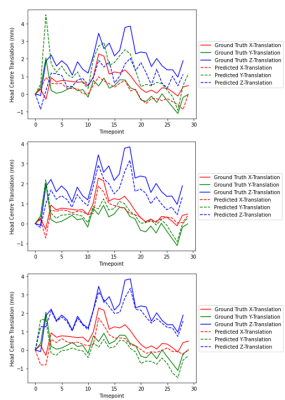
Brain localisation and brain position extraction took 6±1 ms per dataset on Google Colab with GPU enabled. Examples of brain masks generated by the 3D-UNet localisation network are shown in Fig. 2. For the first echo-time, the network successfully localised the brain in 42% of the images, although in 68% of those it also identified a second cluster. For the second echo-time, localisation was successful in 82% of the images, with 46% having a second cluster identified. Lastly, the network successfully extracted the fetal brain in 73% of the images, with 34% of these having a second cluster identified alongside the fetal brain and 4.5% having partial brain coverage by the predicted mask. Fig. 3 shows translational parameters obtained from the localisation network mask vs those estimated with the ground truth segmentations over the 30 dynamics for one fetal subject where localisation was successful at all timepoints and echo times. The average translation errors in the x, y and z directions were [0.49, -1.06, 0.99]mm, [0.18, -0.23, 0.54]mm and [0.41, 0.26, 0.17]mm for TE1, TE2, TE3 respectively, showing a comparable performance for TE2 and TE3. The mean (±SD) displacement error between ground truth and predicted brain position were 1.61(±0.74) mm, 0.65(±0.27) mm and 0.64(±0.80) mm for TE1, TE2 and TE3, respectively.Discussion and Conclusion
This study demonstrates that dynamic 2D-EPI acquisitions contain sufficient information to allow automated fetal brain localisation. The current localisation accuracy varies, with the greater contrast between fetal brain and its surroundings at TE2/TE3 likely responsible for more consistent localisation. Though additional clusters are also localised in a substantial fraction of images, we expect this could be addressed by adding a GAN discriminator network that penalises the network for misclassifying structures other than fetal brain- as previously successfully implemented for multi-label fetal segmentation [7]. Automated centering and zooming of the FOV on the fetal brain could also focus the network and improve performance. Other methods for brain tracking such as based on recurrent neural networks have been proposed [8] and comparison of performance would be valuable. Upon successful localisation, the brain position estimation based on centre of mass displays good accuracy for small brain displacements in a few test subjects, requiring no manual intervention. Brain position estimation was extremely fast on Google Colab and may be faster with a dedicated GPU. For navigator acquisitions, a reduction in acquisition time would be essential: single-TE acquisition with TEs intermediate between TE1 and TE2 (20-30ms) at slightly reduced resolution of 4mm allowing TR<1s are currently being explored. We have demonstrated the potential of 2D-EPI acquisitions, together with dedicated fully-automated neural network processing, to serve as navigator protocol. With an estimated prediction time <10ms, following refinements our method could easily be implemented in a real-time feedback loop, for instance via Gadgetron [9], for prospective motion corrected fetal MRI or MRS.Acknowledgements
This work was supported by the EPSRC Doctoral Training Programme EP/R513064/1, the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project (PIP)), a Sir Henry Wellcome Fellowship, a UKRI FL fellowship and by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.References
[1] A. Uus et al, Deformable Slice-to-Volume Registration for Motion Correction of Fetal Body and Placenta MRI. IEEE Trans Med Imaging, 2020, 39(9): 2750-2759.
[2] W. Bogner et al, 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. NeuroImage, 2014.
[3] M.D. Tisdall et al., Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. MRM, 68(2):389-399, 2012.
[4] B. Gagoski et al, HASTE imaging with EPI volumetric navigators for real-time fetal head motion detection. Proc. Intl. Soc. Mag. Reson. Med. 24, 2016.
[5] P. McDaniel et al, Quantification of Fetal Motion Tracked with Volumetric Navigator MRI Acquisitions. Proc. Intl. Soc. Mag. Reson. Med. 23, 2015.
[6] M. Jenkinson et al, A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 2001, 5: 143-156.
[7] A. Uus et al, 3D UNet with GAN discriminator for robust localisation of the fetal brain and trunk in MRI with partial coverage of the fetal body. NeurIPS, 2020.
[8] A. Singh et al, Deep Predictive Motion Tracking in Magnetic Resonance Imaging: Application to Fetal Imaging. IEEE Trans Med Imaging. 39(11):3523-3534, 2020.
[9] M. S. Hansen et al, Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med, 69: 1768-1776, 2013.
Figures