1812
Assessing the utility of low-resolution images for Hydrocephalus treatment planning1Engineering Science, Penn State University, State College, PA, United States, 2Penn State University, University Park, PA, United States, 3Leiden University Medical Center, Leiden, Netherlands, 4The CURE Children's Hospital of Uganda, Mbale, Uganda, 5Yale, New Haven, CT, United States, 6Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 7University of Toronto, Toronto, ON, Canada
Synopsis
Brain images of a quality lower than is conventionally acceptable may still be useful for planning hydrocephalus treatment. Low-field MRI is a technology of growing global interest which has the capability of producing images of sufficient quality for treatment planning and is affordable enough to disseminate to rural regions of the world. Though deep learning enhancement of low-quality images does improve CNR and apparent quality, spatial errors of brain and CSF after enhanced reconstruction add significant risk to treatment management and should be avoided.
Introduction
Infant Hydrocephalus is the most common neurosurgical condition globally, with over 90% of cases occurring in low- and middle-income countries (LMIC)1,2. Life-saving treatment requires biomedical imaging for planning; however LMIC suffers from lack of access to this expensive and technically challenging technology3-6. Recent advances in low-field MRI (LFMRI) offer the potential for these affordable systems to be disseminated throughout the world for the treatment management of hydrocephalus7-10. Since LFMRI images are inherently of lower quality when compared to the conventional standard, it is important to consider the actual quality threshold required for a given illness prior to clinical use. In the present work, we investigate the utility of reduced-quality and machine learning enhanced images for hydrocephalus treatment planning. While our work is focused on the potential use of emerging low-field MRI technology, the only known image repository of post-infectious infant hydrocephalus is CT based11,12. We developed an image utility assessment which was completed by three senior neurosurgeons with extensive experience in infant hydrocephalus management in LMIC. Using qualitative and quantitative measures, we elucidate the threshold of image quality required for planning hydrocephalus treatment. Additionally, we include an analysis of the risk of using machine learned library information to enhance low quality images.Methods
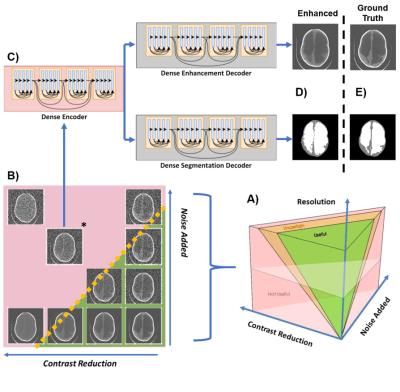
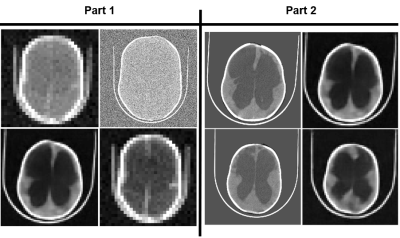
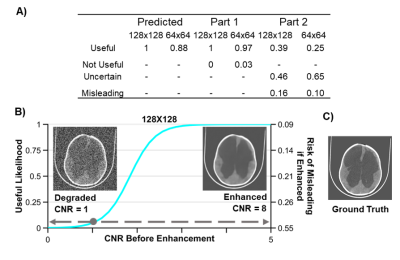
CT images are degraded in terms of resolution, contrast between brain and CSF, and noise. Resolution is adjusted by bilinear interpolation. Contrast between brain and CSF is reduced using a novel approach where the histogram of greyscale values for both tissues are iteratively compressed toward a common mean. Gaussian noise is added with mean equal to variance. Deep learning enhancement is performed using a single-encoder, dual-decoder network13,14. Of the 90 images in the repository, 80 are used for training and the remaining 10 are test images. Enhancement networks are trained for 64x64 and 128x128 images at 7 specific noise and contrast combinations. Part 1 of the assessment includes 420 randomly chosen combinations of degraded images and all 140 deep learning enhanced images. The images were presented in groups of four, including one deep learning enhanced image. Evaluators were asked to indicate which, if any, of the images could be useful for treatment planning. Evaluators were not told there would be enhanced images. Figure 3 shows an example panel from part 1. In part 2 of the assessment, enhanced images deemed useful in part 1 were presented next to their high-resolution versions and evaluators were asked to indicate whether spatial errors in the enhancement reconstruction are acceptable for treatment planning. Logistic regression was used to model the resolution specific likelihood of a degraded image being useful with CNR as the predictor. The same analysis was used for enhanced images to show the predicted risk of misleading treatment due to reconstruction errors when using enhancement.Results
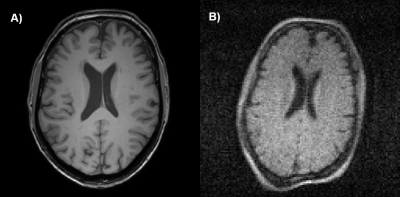
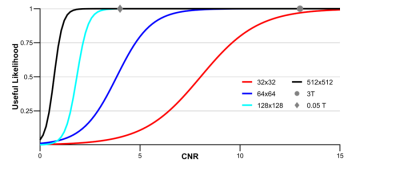
Image resolution and CNR of brain and CSF predict the likelihood of a useful image for hydrocephalus treatment planning as shown in Figure 2. Importantly, Figure 2 also shows that while the brain image from Figure 1 acquired at 3 Tesla has higher resolution, CNR, and cost than the image acquired at 0.05 Tesla, they share the same likelihood of being useful for treatment planning. Deep learning enhancement of low-quality images increases image CNR and apparent usefulness likelihood in 98% of the images shown to evaluators, as can be seen in Figure 3A. However, results from part 2 of the assessment indicate that more than half of the images originally deemed useful had the potential to mislead treatment decisions. The comparison between the usefulness likelihood of a 128x128 degraded image and the risk associated with enhancement of that image is shown in Figure 3B.Discussion
Images with lower quality than is conventionally acceptable can nevertheless be useful for hydrocephalus treatment planning. For 0.05 T brain images with 2 mm resolution (Figure 1B), a CNR of 3 can still provide high likelihood of utility for hydrocephalus treatment planning, as seen in Figure 3. This level of resolution and CNR is within the current capability of low field MRI technology without the use of machine learning enhancement. In the context of global health, this signifies a significant step toward providing state-of-the-art care to rural parts of the world for specific diseases, such as hydrocephalus. Deep learning enhancement of low quality images does provide a significant increase in CNR, however part 2 results suggest that spatial errors resulting from enhancement can be misleading to treatment in a significant number of cases (Figure 4A). Figure 4B further illustrates that for images with resolution relevant to low field MRI, there does not exist a degraded image with low usefulness likelihood that also has low risk of being misleading if enhanced. Because of this we find no scenario where this type of deep learning enhancement may be used without undue risk of misguiding treatment decisions.Conclusion
Lower quality images not customarily considered acceptable by clinicians can still be useful in planning hydrocephalus treatment, but there is substantial risk of misleading structural errors when using deep learning enhancement. These findings advocate for new standards in assessing acceptable image quality for clinical use for specific disease.Acknowledgements
Supported by US National Institutes of Health grant R01HD085853. ClinicalTrials.gov registration number NCT01936272.References
1. Dewan, Michael C., et al. "Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis." Journal of neurosurgery 130.4 (2018): 1065-1079.
2. Warf, Benjamin C. "Educate one to save a few. Educate a few to save many." World neurosurgery 79.2 (2013): S15-e15.
3. World Health Organization. Baseline country survey on medical devices 2010. No. WHO/HSS/EHT/DIM/11.01. World Health Organization, 2011.
4. Klein, Hans-Martin. clinical low field strength magnetic resonance imaging: a practical guide to accessible MRI. Springer, 2015.
5. Malkin, Robert A. "Design of health care technologies for the developing world." Annu. Rev. Biomed. Eng. 9 (2007): 567-587.
6. Gatrad, A. R., S. Gatrad, and A. Gatrad. "Equipment donation to developing countries." Anaesthesia 62 (2007): 90-95.
7. O’Reilly, Thomas, et al. "In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array." Magnetic resonance in medicine 85.1 (2021): 495-505.
8. Sheth, Kevin N., et al. "Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients." JAMA neurology 78.1 (2021): 41-47.
9. Mazurek, Mercy H., et al. "Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage." Nature communications 12.1 (2021): 1-11.
10. Cooley, Clarissa Z., et al. "A portable scanner for magnetic resonance imaging of the brain." Nature biomedical engineering 5.3 (2021): 229-239.
11. Kulkarni, Abhaya V., et al. "Endoscopic treatment versus shunting for infant hydrocephalus in Uganda." New England Journal of Medicine 377.25 (2017): 2456-2464.
12. Paulson, Joseph N., et al. "The Bacterial and Viral Complexity of Postinfectious Hydrocephalus in Uganda." Science translational medicine 12.563 (2020).
13. Cherukuri, Venkateswararao, et al. "Deep MR brain image super-resolution using spatio-structural priors." IEEE Transactions on Image Processing 29 (2019): 1368-1383.
14. Cherukuri, Venkateswararao, et al. "Learning based segmentation of CT brain images: application to postoperative hydrocephalic scans." IEEE Transactions on Biomedical Engineering 65.8 (2017): 1871-1884.
Figures