1803
Assessment of treatment efficacy in T-cell acute lymphoblastic leukemia models with Hyperpolarized MR and NMR metabolomics1Cancer Systems Imaging, MD Anderson Cancer Center, Houston, TX, United States, 2UT MD Anderson Cancer Center UT Health Science Center Houston Graduate School of Biomedical Sciences, Houston, TX, United States, 3Leukemia, MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Metabolic reprogramming is one of the key hallmarks in acquiring aggressive phenotype and chemoresistance in many cancers including T-cell acute lymphoblastic leukemia (T-ALL). To combat chemoresistance, we treated patient-derived xenografts with two metabolic drugs that target two different pathways for T-ALL: IACS-010759, a Complex I inhibitor for OXPHOS pathway and AZD3965, a monocarboxylate transporter-1 (MCT1) inhibitor. Hyperpolarized metabolic imaging in vivo and NMR metabolomics ex vivo was utilized to observe the difference in metabolism with single treatment and in combination. Our results demonstrate that metabolic intervention utilizing OXPHOS blockade can be potentiated by targeting the MCT1 transporter.
Introduction
Metabolic reprogramming is one of the key hallmarks in acquiring aggressive phenotype and chemoresistance in many solid and hematologic cancers including T-cell acute lymphoblastic leukemia (T-ALL). It has been previously demonstrated that T-ALL is dependent on the oxidative phosphorylation (OXPHOS) for energy production1. When this pathway is affected, they shift their metabolic preference to glycolysis and keep proliferating. We posited that a combination therapy to block both the OXPHOS and monocarboxylate transporter-1 (MCT1) can create a novel metabolic synthetic lethality that could be exploited to overcome chemoresistance in eradicate T-ALL. We employed two well-studied inhibitors: IACS-010759, a Complex I inhibitor for OXPHOS pathway2 and AZD3965, an MCT1 inhibitor3. To test the efficacy of these metabolic inhibitors and determine how metabolism is impacted, hyperpolarized magnetic resonance was employed for real-time metabolic imaging. With metabolic HP-MR, the dynamic biochemical conversion can be monitored between pyruvate and lactate in vivo with over 10,000 fold sensitivity enhancement over conventional MRI4. Nuclear Magnetic Resonance (NMR) metabolic profiling of ex vivo spleen samples where T-ALL cells accumulate, was performed to compare the dynamic metabolism to the total metabolism.Methods
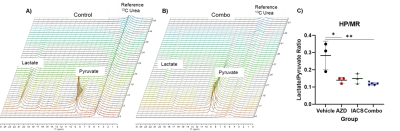
Hyperpolarized 1-13C Pyruvate MRS was employed to study the metabolic processes in different patient-derived leukemia mouse models. There were four different mouse cohorts, a vehicle control group with no treatment, one group that was only treated with IACS-010759 for 30 minutes, one group treated with only AZD3965 for 24 hours, and a group with a combination treatment with both the drugs with their respective times. The dissolution DNP (HyperSense, Oxford Instruments, UK) operating at 3T was utilized to hyperpolarize 1-13C pyruvate. The 13C magnetic resonance spectra of hyperpolarized 1-13C pyruvate were acquired at 7T Bruker MRI scanner. After imaging, the spleen was removed for each mouse and flash frozen for NMR metabolomics. The spleens were pulverized, and the metabolites were extracted with a 2:1 Methanol: Water solution. The solvent was removed, and the residue was dissolved with D2O and NMR analysis was conducted.Results/Discussion
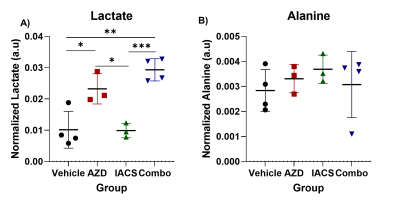
The pyruvate-to-lactate conversion decreases in all treatment groups, this was demonstrated by the lactate over pyruvate ratios, higher the ratio the more lactate production. Vehicle ratio average was 0.25 while treatments with AZD3965 and IACS-010759 decreased the ratio, 0.14 and 0.15 respectively (Figure 1). With the combination group, the ratios slightly decreased compared to the single treated groups, but significantly lower compared to the vehicle control group demonstrating greater efficacy of the combination treatment over monotherapies. Furthermore, the NMR metabolomics data demonstrates a different angle of the metabolic alterations associated with each treatment (Figure 2). In the case of the vehicle and IACS-010759 treated groups, the lactate levels were comparable with each other. In AZD3965 and the combination groups, lactate levels were higher than the other groups, which correlates with the inhibition of the MCT1 transporter. With the monocarboxylate transporter being blocked, there is an accumulation of lactate as lactate cannot be transported out of the cell leading to accumulation, which is supported by our metabolomics profiling. On the other hand, alanine levels were comparable across all the groups.Conclusion
These results demonstrate a novel synthetic vulnerability of concomitant blockade of OXPHOS and MCT-1 which may translate into successful therapies in T-ALL and OXPHOS-dependent malignancies. HP-MRS technique can assess the efficacy of therapy in real-time in T-ALL and may help physicians in the management of chemoresistance in T-ALL. Mechanistically, inhibition of MCT1 by AZD3965 therapy in leukemia-bearing mice led to lactate accumulation, while Complex I blockade resulted in upregulation of MCT-1; consequently, combinatorial therapy caused complete mitochondria shut-down and drastic inhibition of tumor growth both in vitro and in vivo in the patient-derived xenograft model.Acknowledgements
This research was funded in part by a grant from Department of Defense PC131680; Cancer Prevention and Research Institute of Texas (CPRIT; RP180164); Duncan Family Institute for Cancer Prevention and Risk Assessment and by Institutional Research Grants from MD Anderson Cancer Center; by grants from the US National Cancer Institute (U01 CA214263, U54 CA151668 and R21 CA185536, R01 CA218004; and 1P50 CA221707-01). This work also was supported by the National Institutes of Health/NCI Cancer Center Support Grant under award number P30 CA016672.References
1. Baran N, Lodi A, Sweeney SR et al., Mitochondrial Complex I Inhibitor IACS-010759 Reverses the NOTCH1-Driven Metabolic Reprogramming in T-ALL Via Blockade of Oxidative Phosphorylation: Synergy with Chemotherapy and Glutaminase Inhibition. Blood, 2018, 132 (Supplement 1): 4020.
2. Molina JR, Sun Y, Protopopova M et al., An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med, 2018, 24: 1036-1046.
3. Grønningsæter IS, Reikvam H, Aasebø E et al.,Targeting Cellular Metabolism in Acute Myeloid Leukemia and The Role of Patient Heterogeneity. Cells, 2020, 9(5):E1155.
4. Dutta P, Perez MR, Lee J et al. Combining Hyperpolarized Real-Time Metabolic Imaging and NMR Spectroscopy To Identify Metabolic Biomarkers in Pancreatic Cancer. J Proteome Res, 2019, 18: 2826-2834.
Figures