1800
Improving the biocompatibility of parahydrogen hyperpolarized [1-13C]pyruvate1Molecular Biotechnology, University of Torino, Torino, Italy, 2Institute of Biostructure and Bioimaging, Nation Research Council, Torino, Italy
Synopsis
ParaHydrogen Induced Polarization is a hyperpolarization method much less technically demanding and affordable than d-DNP. The Side Arm Hydrogenation method allowed to obtain hyperpolarized [1-13C]pyruvate that can be used for metabolic studies, but concerns about the safety and bio-compatibility of the final aqueous solution of the HP products may be present, due to organic solvents and metal complex. In this work, a method to remove all the traces of toxic solvents and metal complex from the final product will be presented, together with the 13C-MR images obtained using the metabolite thus hyperpolarized.
Introduction
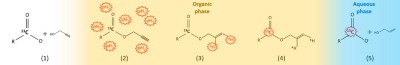
Hyperpolarized [1-13C]pyruvate is under intense study for the in-vivo metabolic investigation of different pathologies [1]. Important limitations to the exploitation of this powerful diagnostic tool are the high cost of the d-DNP technology and the technical challenges intrinsic to this technique. ParaHydrogen Induced Polarization is much less technically demanding and is affordable. The Side Arm Hydrogenation method (figure 1) [2] allowed to obtain hyperpolarized [1-13C]pyruvate that has been used for metabolic studies [3]. However, concerns about the safety and bio-compatibility of the final aqueous solution of the HP products may be present. These solutions still contain traces of organic solvents and catalyst, that are used for the parahydrogen hyperpolarization procedure. In this work, a method to remove all the traces of toxic solvents and metal complex from the final product will be presented, together with the 13C-MR images obtained using the metabolite thus hyperpolarized.Methods
[1- 13C] pyruvate was hyperpolarized by means hydrogenation, using parahydrogen, of its propargylic ester (propargyl- [1-13C]pyruvate)in an organic, hydrophobic solvent (chloroform or toluene/ethanol), in order to allow the application of the phase extraction method. Spin order transfer from the parahydrogen protons to the 13C carboxylate spin has been carried out by means of magnetic field cycling and cleavage of the ester has been obtained using and aqueous base solution (NaOH 0.1M). The hyperpolarized metabolite is extracted in the water phase, while the catalyst is retained in the organic one, and the two phases separate in few seconds. An acidic buffer (HEPES) is added to the aqueous phase, in order to reach physiological pH, the aqueous solution is, then, filtered through a lipophilic resin ( TENAX® TA porous polimer adsorbent), collected in a syringe for MRI in-vitro and in-vivo. 13C-MRS and 13C-csi experiments have been acquired on a 7T-Bruker MRI machine.Results
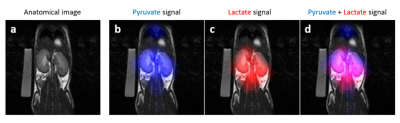
The aqueous solution of hyperpolarized [1-13C]pyruvate, before filtration through the lipophilic resin, contains non-neglectable concentrations of the organic solvents (chloroform and toluene) that have been used to carry out the parahydrogenation reaction. Cytotoxicity studies carried out on tumor cells have shown that a toxicity effect associated with the presence of the organic solvent (chloroform). Filtration through the lipophilic resin allows to remove the solvents almost completely, and the concentration of these solvents is lower than those recommended by the EPA (Environmental Protection Agency). Most importantly, the 13C hyperpolarization level is still sufficiently high to allow the acquisition of MR images in-vitro and in-vivo (figure 2). In the in-vivo experiments 13C-csi clearly shows the signals from HP [1-13C] lactate derived from metabolism of HP [1-13C]pyruvate, in a mouse.Discussion and conclusions
Filtration of the aqueous solution of hyperpolarized [1-13C] pyruvate through a lipophilic resin (Tenax® TA) led to the complete removal of the organic solvents, while the 13C hyperpolarization on the product is left almost unaffected, still sufficient for 13C MRI studies. The attainment of a fully biocompatible solution of hyperpolarized metabolite, free from toxic solvents and metal complexs, makes parahydrogen hyperpolarized [1-13C]pyruvate an effective probe for metabolic studies in-vivo.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie (Grant Agreement No. 766402) and the FETOPEN program (Grant agreement 858149, proposal acronym Alternatives to Gd). The Italian MIUR is also acknowledged for funding (PON Research and Innovation 2014-2020, CUP ARS01_00144, Novel molecular Imaging methods for the investigation of oncological and neurodegenerative diseases, MOLIM OncoBrain).References
[1] E.M. Serrão, K.M. Brindle, Potential Clinical Roles for Metabolic Imaging with Hyperpolarized [1-13C]Pyruvate, Front. Oncol. 6 (2016) 59. doi:10.3389/fonc.2016.00059.
[2]F. Reineri, T. Boi, S. Aime, ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate, Nat. Commun. 6 (2015) 5858. doi:10.1038/ncomms6858.
[3]E. Cavallari, C. Carrera, G. Di Matteo, O. Bondar, S. Aime, F. Reineri, In-vitro NMR Studies of Prostate Tumor Cell Metabolism by Means of Hyperpolarized [1-13C]Pyruvate Obtained Using the PHIP-SAH Method, Front. Oncol. 10 (2020) 1–9. doi:10.3389/fonc.2020.00497.
Figures