1798
Dissolved-phase 129Xe longitudinal relaxation times at low and high magnetic field strengths1University of North Carolina Chapel Hill, Chapel Hill, NC, United States
Synopsis
We provide longitudinal relaxation measurements of hyperpolarized (HP) 129Xe gas dissolved in a variety of solvents at both low and high magnetic field strengths. The method of measuring T1 presented here is less sensitive than more commonly used methods to RF flip angle miscalibration, which has caused the significant variation in dissolved-phase 129Xe T1 values reported in the past. A field dependent study of gas depolarization by hollow membrane fibers commonly used to dissolve xenon in blood or other solvents is also included, which provides insight to experimental limitations on their use in both field regimes.
Introduction
In the past, in vitro T1 relaxation measurements of 129Xe in solvents like water, vegetable oils, and blood, have served as a first step in assessing the feasibility of dissolved-phase HP 129Xe MRI.1 For water and oil the T1 relaxation times have been determined to be on the order of tens of seconds, enabling both HP 129Xe spectroscopy and imaging in organs like the brain, kidney, distal tumors, and adipose tissue. Because the delivery of HP 129Xe throughout the body depends on blood circulation time, the T1 of xenon in blood is also highly relevant to in vivo dissolved-phase HP 129Xe studies. To measure the T1 of xenon in blood, the polarized gas has to be gently infused in blood to avoid the creation of paramagnetic foams which cause rapid depolarization.2,3 This can be accomplished by either mixing the blood sample with saline that has been pre-saturated with HP 129Xe, or by directly infusing HP 129Xe in blood using membrane exchange modules.3-5Here, to assess the feasibility of dissolved-phase xenon at low field, we present T1 relaxation measurements of 129Xe dissolved in corn oil, deionized (DI) water, and blood plasma at low field strengths (2.1 mT). Similar measurements were also performed at high (11.7 T) magnetic field strengths, for which significantly different dissolved-phase 129Xe T1 values have been reported.1-3,5,7 Additionally, we investigate the field dependence of HP 129Xe depolarization in membrane exchange modules commonly used to infuse HP 129Xe in blood. The results obtained outline significant experimental design concerns when working with these membranes at both low and high magnetic field strengths.
Methods
A home-built spectrometer was used to measure the longitudinal relaxation of 129Xe dissolved in water, corn oil, and blood plasma at 2.1 mT.8 For these measurements, a gas mixture containing 70% HP 129Xe gas (P~20% using a Polarean 9800 Xe polarizer) and 30% N2, was bubbled in the sample for 10 seconds to reach saturation. A variable delay, with millisecond precision, was implemented between the end of gas delivery and RF excitation to allow for longitudinal relaxation to occur. Simultaneous detection of the gas phase signal and dissolved-phase signal enabled correction for gas-phase relaxation of the xenon gas reservoir housed in the fringe field of the magnet. For the high field measurements, the same procedure was followed. In this case, measurements were made on a high-resolution Varian NMR spectrometer, after HP 129Xe gas was directly bubbled into the NMR tube. Again, a variable delay was implemented between the end of HP 129Xe delivery and RF excitation to allow for longitudinal relaxation to occur. Consecutive RF pulse were used to destroy any remaining magnetization before the subsequent measurement was taken.To measure 129Xe depolarization in the exchange module (3M Liqui-Cel SP-0.5x1 Series Membrane Contactor G591), HP 129Xe was flown trough the exchange module, placed in various locations where the magnetic field ranged from 2.1 mT to 9 T, before the magnetization of the exhaust gas was probed with a single 90° excitation pulse.
Results
Longitudinal relaxation measurements at 2.1 mT returned T1 values for 129Xe dissolved in DI water, corn oil, and blood plasma of 49, 11, and 19 s, respectively. To our knowledge, these are the first T1 values reported for 129Xe dissolved in these solvents at low field strengths. At 11.7 T, T1 values of 129Xe dissolved in DI water, corn oil, blood plasma, saline, and 20% intralipid were found to be 97, 16, 20, 72, and 18 s, respectively. The difference between our values and previously reported values of xenon dissolved in water and corn oil at high field strengths, can be easily explained by the high sensitivity of the small flip angle methods, widely used to measure HP 129Xe T1 at high field, to the accuracy of the calibration of the RF flip angle and magnitude of the T1 value, which can be summarized by the equation:$$\frac{T_1-T_1'}{T_1T_1'} = \frac{1}{\tau}\ln(\frac{\cos\theta'}{\cos\theta})$$
where T1 and T1’ are the longitudinal relaxation values obtained for two different RF flip angles. The membrane exchange module study revealed significant (> 30%) gas depolarization even at a field strength of 1.5 T. The field-dependent depolarization results obtained here for xenon diffusing through these porous membranes agrees well with our current theoretical understanding of HP 129Xe surface relaxation and its field dependence.10,11 The relaxation that xenon undergoes while diffusing across these exchange membranes not only prevents their use at low field strengths, but also suggests that for high field studies these membrane modules need to be located as close as possible to the magnet isocenter, not only to reduce transfer time, but to prevent significant xenon relaxation through the membranes.
Conclusion
Dissolved-phase 129Xe T1 values obtained at low field are only slightly lower than the values obtained at high field. While these values support the feasibility of dissolved-phase xenon studies in the low field regime, for in vivo dissolved-phase studies the T1 of xenon in blood will be the limiting factor. An exact measurement of this value at low field was attempted unsuccessfully as the use of membrane fibers is evidently not an option at lower field strengths due to excessive depolarization of the gas.Acknowledgements
This work was partially supported by the NIH grant number R01DK108231, R01DK123206, and R21EB031319; Dr. Branca’s startup fund; and the NSF Graduate Research Fellowship DGE-1650116.References
1. M. Albert, V. Schepkin, T. B. Measurement of 129Xe T1 in Blood to Explore the Feasibility of Hyperpolarized 129Xe MRI. J. Comput. Assist. Tomogr. 19, 975–978 (1995).
2. Wolber, J., Cherubini, A., Dzik-Jurasz, A. S. K., Leach, M. O. & Bifone, A. Spin-lattice relaxation of laser-polarized xenon in human blood. Proc. Natl. Acad. Sci. U. S. A. 96, 3664–3669 (1999).
3. Norquay, G. et al. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn. Reson. Med. 74, 303–311 (2015).
4. Baumer, D., Brunner, E., Blümler, P., Zänker, P. P. & Spiess, H. W. NMR spectroscopy of laser-polarized 129Xe under continuous flow: A method to study aqueous solutions of biomolecules. Angew. Chemie - Int. Ed. 45, 7282–7284 (2006).
5. Cleveland, Z. I., Möller, H. E., Hedlund, L. W. & Driehuys, B. Continuously Infusing Hyperpolarized 129 Xe into Flowing Aqueous Solutions Using Hydrophobic Gas Exchange Membranes. Journal of Physical Chemistry B vol. 113 12489–12499 (2009).
6. Norquay, G., Leung, G., Stewart, N. J., Wolber, J. & Wild, J. M. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magn. Reson. Med. 77, 1399–1408 (2017).
7. Bifone, A. et al. NMR of laser-polarized xenon in human blood. Proc. Natl. Acad. Sci. U. S. A. 93, 12932–12936 (1996).
8. Bryden, N., Antonacci, M., Kelley, M. & Branca, R. T. An open-source, low-cost NMR spectrometer operating in the mT field regime. J. Magn. Reson. 332, 107076 (2021).
9. Nouls, J. A Constant-Volume Ventilator and Gas Recapture System for Hyperpolarized Gas MRI of Mouse and Rat Lungs. Concepts Magn. Reson. Part B Magn. Reson. Eng. 49B, 78–88 (2011).
10. A. Abragam, Principles of Nuclear Magnetism. Oxford University Press, New York (1961).
11. Driehuys, B., Cates, G. D. & Happer, W. Surface Relaxation Mechanisms of Laser-Polarized 129Xe. Phys. Rev. Lett. 74, 4943–4946 (1995).
Figures
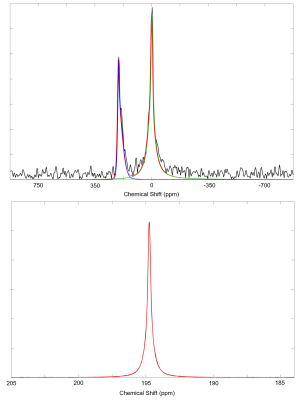
Figure 1: Examples of data and fits collected at 2.1 mT (top) and 11.7 T (bottom). The dissolved-phase signal appears approximately 200 ppm downfield from the gas-phase signal (off screen for high field data) for all solvents used in this study. Both low and high field data is fit to a pseudo-Voigt distribution.
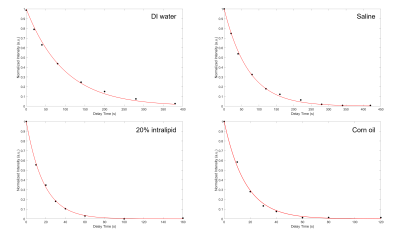
Figure 2: High field data showing the exponential decay of the signal intensity as a function of the time delay between gas delivery and signal acquisition. The fit returns T1 values for 129Xe dissolved in DI water, saline, and 20% intralipid, and corn oil of 97.1, 72.4, and 18.3 s, and 16.2 s, respectively. Intensities were acquired by peak integration of the fits seen in Fig. 1 and are normalized to the gas peak intensity to correct for gas relaxation in the Tedlar bag placed in the fringe field of the magnet.
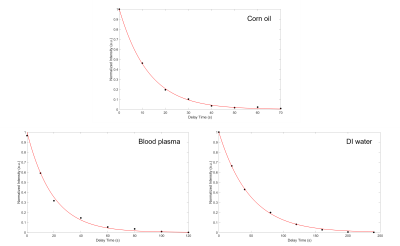
Figure 3: Low field data showing the exponential decay of the magnetization as a function of the time delay between gas delivery and signal acquisition. The fits return T1 values for 129Xe dissolved in corn oil, blood plasma, and DI water of 11.8, 19.2, and 49.2 s, respectively. Intensities were acquired by peak integration of the fits seen in Fig. 1 and are normalized to the gas peak intensity to correct for the relaxation of the gas in the Tedlar bag where the HP 129Xe gas was stored.
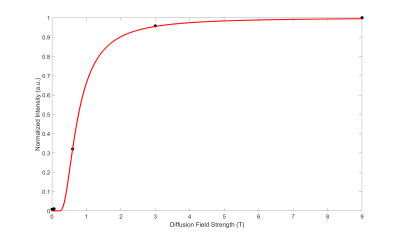
Figure 4: Field dependence of 129Xe relaxation in the exchange membranes. Spectra were acquired using the same setup and by flowing the same amount of HP 129Xe gas through the membrane module, placed in different field locations. Data was collected in order of ascending field strength to rule out gas relaxation in the reservoir. The signal intensity was then normalized to the signal collected with the diffusion membrane placed close to the magnet isocenter. Data closely follows the expected theoretical magnetic field-dependency expected for the 129Xe surface relaxation (red line).